Abstract
Background
We investigated whether model-based indices of cerebral autoregulation (CA) are associated with outcomes after pediatric traumatic brain injury.
Methods
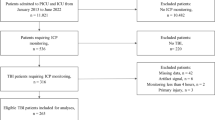
This was a retrospective analysis of a prospective clinical database of 56 pediatric patients with traumatic brain injury undergoing intracranial pressure monitoring. CA indices were calculated, including pressure reactivity index (PRx), wavelet pressure reactivity index (wPRx), pulse amplitude index (PAx), and correlation coefficient between intracranial pressure pulse amplitude and cerebral perfusion pressure (RAC). Each CA index was used to compute optimal cerebral perfusion pressure (CPP). Time of CPP below lower limit of autoregulation (LLA) or above upper limit of autoregulation (ULA) were computed for each index. Demographic, physiologic, and neuroimaging data were collected. Primary outcome was determined using Pediatric Glasgow Outcome Scale Extended (GOSE-Peds) at 12 months, with higher scores being suggestive of unfavorable outcome. Univariate and multiple linear regression with guided stepwise variable selection was used to find combinations of risk factors that can best explain the variability of GOSE-Peds scores, and the best fit model was applied to the age strata. We hypothesized that higher GOSE-Peds scores were associated with higher CA values and more time below LLA or above ULA for each index.
Results
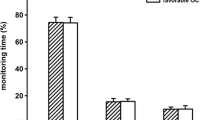
At the univariate level, CPP, dose of intracranial hypertension, PRx, PAx, wPRx, RAC, percent time more than ULA derived for PAx, and percent time less than LLA derived for PRx, PAx, wPRx, and RAC were all associated with GOSE-Peds scores. The best subset model selection on all pediatric patients identified that when accounting for CPP, increased dose of intracranial hypertension and percent time less than LLA derived for wPRx were independently associated with higher GOSE-Peds scores. Age stratification of the best fit model identified that in children less than 2 years of age or 8 years of age or more, percent time less than LLA derived for wPRx represented the sole independent predictor of higher GOSE-Peds scores when accounting for CPP and dose of intracranial hypertension. For children 2 years or younger to less than 8 years of age, dose of intracranial hypertension was identified as the sole independent predictor of higher GOSE-Peds scores when accounting for CPP and percent time less than LLA derived for wPRx.
Conclusions
Increased dose of intracranial hypertension, PRx, wPRx, PAx, and RAC values and increased percentage time less than LLA based on PRx, wPRx, PAx, and RAC are associated with higher GOSE-Peds scores, suggestive of unfavorable outcome. Reducing intracranial hypertension and maintaining CPP more than LLA based on wPRx may improve outcomes and warrants prospective investigation.


Similar content being viewed by others
References
Hawley CA, Ward AB, Long J, Owen DW, et al. Prevalence of traumatic brain injury amongst children admitted to hospital in one health district: a population based study. Injury. 2003;354:256–60.
Kochanek PM, Tasker RC, Carney N, Totten AM, et al. Guidelines for the management of pediatric severe traumatic brain injury, third edition: update of the brain trauma foundation guidelines. Pediatr Crit Care Med. 2019;20(3S Suppl 1):S1–82.
Maas AIR, Menon DK, Adelson PD, Andelic N, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048.
Donnelly J, Budohoski KP, Smielewski P, Czosnyka M. Regulation of the cerebral circulation: bedside assessment and clinical implications. Crit Care. 2016;20(1):129.
Mehta A, Kochanek PM, Tyler-Kabara E, Adelson PD, et al. Relationship of intracranial pressure and cerebral perfusion pressure with outcome in young children after severe traumatic brain injury. Dev Neurosci. 2010;32(5–6):413–9.
Steiner LA, Czosnyka M, Piechnik SK, Smielewski P, et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30:7338.
Liu X, Maurits NM, Aries MJH, Czosynka M, Ercole A, Donnelly J, et al. Monitoring of optimal cerebral perfusion pressure in traumatic brain injured patients using a multi-window weighting algorithm. J Neurotrauma. 2017;34:3081–8.
Liu X, Donnelly J, Czosnyka M, Aries MJH, et al. Cerebrovascular pressure reactivity monitoring using wavelet analysis in traumatic brain injury patients: a retrospective study. PLoS Med. 2017;14(7):e1002348.
Zeiler FA, Donnelly J, Calviello L, Lee JK, et al. Validation of pressure reactivity and pulse amplitude indices against the lower limit of autoregulation, part I: experimental intracranial hypertension. J Neurotrauma. 2018;35(23):2803–11.
Zeiler FA, Donnelly J, Menon DK, Smielewski P, et al. A description of a new continuous physiological index in traumatic brain injury using the correlation between pulse amplitude of intracranial pressure and cerebral perfusion pressure. J Neurotrauma. 2018;35:963–74.
Kochanek PM, Carney N, Adelson PD, Ashwal S, et al. Guidelines for the acute management of severe traumatic brain injury in infants, children and adolescents—second edition. Pediatr Crit Care Med. 2012;13(Suppl 1):S1–82.
Lazardis C, Desantis SM, Smielewski P, Menon DK, Hutchinson P, Pickard JD, et al. Patient-specific thresholds of intracranial pressure in severe traumatic brain injury. J Neurosurg. 2014;120(4):893–900.
Zeiler FA, Donnelly J, Smielewski P, Menon DK, Hutchinson J, Czosynka M. Critical thresholds of intracranial pressure-derived continuous cerebrovascular reactivity indices for outcome prediction in noncraniectomized patients with traumatic brain injury. J Neurotrauma. 2018;35(10):1107–15.
Donnelly J, Czosnyka M, Adams H, Robba C, et al. Individualizing thresholds of cerebral perfusion pressure using estimated limits of autoregulation. Crit Care Med. 2017;45(9):1464–71.
Czosnyka M, Czosynka Z, Smelewski P. Pressure reactivity index: journey through the past 20 years. Acta Neurochir. 2019;519:2063–5.
Appavu B, Foldes S, Burrows BT, Jacobson A, Abruzzo T, Boerwinkle V, Willyerd A, Mangum T, Gunnala V, Marku I, Adelson PD. Multimodal assessment of cerebral autoregulation and autonomic function after pediatric cerebral arteriovenous malformation rupture. Neurocrit Care. 2021;34(2):537–46.
Hiler M, Czosnyka M, Hutchinson P, Balestreri M, et al. Predictive value of initial computerized tomography scan, intracranial pressure, and state of autoregulation in patients with traumatic brain injury. J Neurosurg. 2006;104:731–7.
Sgouros S, Goldin JH, Hockley AD, Wake MJC, Natarajan K. Intracranial volume change in childhood. J Neurosurg. 1999;91:610–6.
Beers SR, Wisniewski SR, Garcia-Filion P, Tian Y, Hahner T, Berger RP, et al. Validity of a pediatric version of the glagow outcome scale—extended. J Neurotrauma. 2012;29(6):1126–39.
Portet S. A primer on model selection using the Akaike information criterion. Infect Dis Model. 2020;5:111–28.
Suzuki K. The changes of regional cerebral blood flow with advancing age in normal children. Nagoya Med J. 1990;34:159–70.
Bode H, Wais U. Age dependence of flow velocities in basal arteries. Arch Dis Child. 1998;63(6):606–11.
Wintermark M, Cotting D, Roulet E, van Melle G, Meuli R, Maeder P, et al. Brain perfusion in children: evolution with age assessed by quantitative perfusion computed tomography. Pediatrics. 2004;113(6):1642–52.
Chiron C, Raynaud C, Maziere B, et al. Changes in regional cerebral blood flow during brain maturation in children and adolescents. J Nucl Med. 1992;33:696–703.
Vavilala MS, Kincaid MS, Muangman SL, Suz P, Roet I, Lam AM. Gender differences in cerebral blood flow velocity and autoregulation between the anterior and posterior circulations in healthy children. Pediatr Res. 2005;58:574–8.
Woods KS, Horvat CM, Kantawala S, Simon DW, Rakkar J, Kochanek PK, et al. Intracranial and cerebral perfusion pressure thresholds associated with in hospital mortality across pediatric neurocritical care. Pediatr Crit Care Med. 2021;22(2):135–46.
Brady KM, Shaffner DH, Lee JK, Easley RB, et al. Continuous monitoring of cerebrovascular pressure reactivity after traumatic brain injury in children. Pediatrics. 2009;124(6):e1205–12.
Lewis PM, Czosnyka M, Carter BG, Rosenfeld JV, et al. Cerebrovascular pressure reactivity in children with traumatic brain injury. Pediatr Crit Care Med. 2015;16(8):739–49.
Zeiler FA, Ercole A, Czosnyka M, Smielewski P, et al. Continuous cerebrovascular reactivity monitoring in moderate/severe traumatic brain injury: a narrative review of advances in neurocritical care. Brit J Anesth. 2020;124(4):440–53.
Akerlund CA, Donnelly J, Zeiler FA, Helbok R, Holst A, Cabeleira M, et al. Impact of duration and magnitude of raised intracranial pressure on outcome after severe traumatic brain injury: a CENTER-TBI high resolution group study. PLOS ONE. 2020;15(12):e0243427.
Akhondi-Asl A, Vonberg FW, Au CC, Tasker RC. Meaning of intracranial pressure-to-blood pressure fisher-transformed Pearson correlation-derived optimal cerebral perfusion pressure: testing empiric utility in a mechanistic model. Crit Care Med. 2018;46(12):e1160–6.
Ursino M. A mathematical study of human intracranial hydrodynamics. Part 1—the cerebrospinal fluid pulse pressure. Ann Biomed Eng. 1988;16:379–401.
Ursino M. A mathematical study of human intracranial hydrodynamics. Part 2—simulation of clinical tests. Ann Biomed Eng. 1988;16:403–16.
Funding
This work was funded in part by the United States Department of Defense Congressionally Directed Medical Research Programs Epilepsy Research Program (W81XWH-19-1-0514).
Author information
Authors and Affiliations
Contributions
BA, MT, SF, and PDA contributed to conception and design of this study. BA, MT, SF, BB, MK, AJ, and PDA contributed to acquisition and analysis of data and drafting a significant portion of the article and figures. All authors approved the final draft of this manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
Dr. Appavu previously received a completed research grant from Moberg ICU Solutions, outside the scope of this work. All other authors do not have relevant conflicts of interest.
Ethical Approval/Informed Consent
This study was performed under all ethical research guidelines at Phoenix Children’s Hospital, and the institutional review board (#19-284) at Phoenix Children’s Hospital approved this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is related to the Invited Commentary available at https://link.springer.com/article/10.1007/s12028-021-01307-z.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplement 1
Age-stratified demographic and classic physiologic data. n, count; %, percent; CT, computed tomography; IQR, interquartile ratio; PRISM, Pediatric Risk of Mortality; mm, millimeters; mmHg, millimeters of mercury; ICP, intracranial pressure; CPP, cerebral perfusion pressure; ABP, arterial blood pressure; HR, heart rate; bpm, beats per minute; GOSE-Peds, Glasgow Outcome Scale Extended-Pediatrics. (DOCX 15 KB)
Supplement 2
Age-stratified characteristics of model-based indices of cerebral autoregulation. n, count; IQR, interquartile ratio; mmHg, millimeters of mercury; %, percent; PRx, pressure reactivity index; PAx, pulse amplitude index; wPRx, wavelet pressure reactivity index; RAC, correlation coefficient of intracranial pressure pulse amplitude and cerebral perfusion pressure; CPPOpt, optimal cerebral perfusion pressure; LLA, lower limit of autoregulation; ULA, upper limit of autoregulation. (DOCX 18 KB)
Supplement 3
Sex-based differences in model-based indices of cerebral autoregulation. SD, standard deviation; PRx, pressure reactivity index; PAx, pulse amplitude index; wPRx, wavelet pressure reactivity index; RAC; correlation coefficient of intracranial pressure pulse amplitude and cerebral perfusion pressure; %, percent; LLA, lower limit of autoregulation; ULA, upper limit of autoregulation. (DOCX 14 KB)
Supplement 4
Age-stratified univariate analysis of the association of demographic, neuroimaging and physiologic data with GOSE-Peds scores, 12 months post injury. Bold represent significant variables as related to global functional outcome determined by GOSE-Peds, Glasgow Outcome Scale Extended-Pediatrics (GOSE-Peds). RMSE, root mean square error; GCS, Glasgow Coma Scale; PRISM, pediatric risk of mortality; CT, computed tomography; dICH, dose of intracranial hypertension; CPP, cerebral perfusion pressure; ABP, arterial blood pressure; HR, heart rate; mmHg, millimeters of mercury; bpm, beats per minute; PRx, pressure reactivity index; PAx, pulse amplitude index; wPRx, wavelet pressure reactivity index; RAC; correlation coefficient of intracranial pressure pulse amplitude and cerebral perfusion pressure; %, percent; LLA, lower limit of autoregulation; ULA, upper limit of autoregulation. (DOCX 30 KB)
Rights and permissions
About this article
Cite this article
Appavu, B., Temkit, M., Foldes, S. et al. Association of Outcomes with Model-Based Indices of Cerebral Autoregulation After Pediatric Traumatic Brain Injury. Neurocrit Care 35, 640–650 (2021). https://doi.org/10.1007/s12028-021-01279-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01279-0