Abstract
Background/objective
In November 2014, our Neurointensive Care Unit began a multi-phased progressive early mobilization initiative for patients with subarachnoid hemorrhage and an external ventricular drain (EVD). Our goal was to transition from a culture of complete bed rest (Phase 0) to a physical and occupational therapy (PT/OT)-guided mobilization protocol (Phase I), and ultimately to a nurse-driven mobilization protocol (Phase II). We hypothesized that nurses could mobilize patients as safely as an exclusively PT/OT-guided approach.
Methods
In Phase I, patients were mobilized only with PT/OT at bedside; no independent time out of bed occurred. In Phase II, nurses independently mobilized patients with EVDs, and patients could remain out of bed for up to 3 h at a time. Physical and occupational therapists continued routine consultation during Phase II.
Results
Phase II patients were mobilized more frequently than Phase I patients [7.1 times per ICU stay (± 4.37) versus 3.0 times (± 1.33); p = 0.02], although not earlier [day 4.9 (± 3.46) versus day 6.0 (± 3.16); p = 0.32]. All Phase II patients were discharged to home PT services or acute rehabilitation centers. No patients were discharged to skilled nursing or long-term acute care hospitals, versus 12.5% in Phase I. In a multivariate analysis, odds of discharge to home/rehab were 3.83 for mobilized patients, independent of age and severity of illness. Other quality outcomes (length of stay, ventilator days, tracheostomy placement) between Phase I and Phase II patients were similar. No adverse events were attributable to early mobilization.
Conclusions
Nurse-driven mobilization for patients with EVDs is safe, feasible, and leads to more frequent ambulation compared to a therapy-driven protocol. Nurse-driven mobilization may be associated with improved discharge disposition, although exact causation cannot be determined by these data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immobility during hospitalization leads to problems including muscle breakdown, bone resorption, cardiac and pulmonary complications, deep vein thrombosis (DVT), pulmonary embolus, and long-term cognitive and physical impairments [1,2,3,4,5]. Intensive Care Unit (ICU)-acquired weakness can begin within 24 h of ICU admission and continue to evolve throughout hospitalization [6]. It may take years after discharge to reach peak functional recovery [7, 8]. The benefits of early mobilization have been recognized in recent literature and include improved peripheral and respiratory muscle strength [7], increased quality of life, earlier ventilator liberation, decreased incidence of delirium, and greater functional independence [9,10,11].
Mobilization in general critical care may include active or passive range of motion, seated balance activities, transfer training, and walking [3]. Although a clear definition of “early mobilization” is lacking, examples of early mobilization in the Neuro ICU include: sitting on the edge of the bed, progressing to a stand-pivot position, transferring out of bed to a chair position, ambulating, increasing independence with activities of daily living (ADLs), and maintaining joint integrity [12]. Despite evidence of the benefit, safety, and feasibility of early ICU mobilization, multiple factors in the neuroscience population, such as high fall risk and impulsivity, may complicate patient mobilization. Patients with aneurysmal subarachnoid hemorrhage (SAH) represent a unique patient population in the Neuro ICU, as these patients may be at particular risk for immobility due to concerns of exacerbating delayed cerebral ischemia (DCI) and/or dislodging external ventricular drains (EVD). Because of these concerns, our institution had historically taken a conservative approach toward mobilizing patients with an EVD. Patients who had an EVD were on strict bed rest until the drain was removed. While on strict bed rest, patients were not eligible for physical therapy (PT) evaluation, and out-of-bed activity was not permitted. The primary hypothesis of our early mobilization initiative was that implementation of a nurse-driven early mobilization protocol would lead to earlier and more frequent mobilization sessions.
Methods
Our early mobilization initiative represents a prospective observational cohort study with one historical cohort (Phase 0). The project spanned three distinct phases (Phase 0, I, and II) between 2013 and 2016. For the purposes of the study, the term, ‘mobilization session’ referred to patient activity that occurred either seated at the edge of the bed or out of bed. The aim of the study was to determine whether a nurse-driven mobilization protocol would result in safe and more frequent mobilization than institutional standard care. This mobilization protocol was implemented as standard of care rather than as a formal research study. As such, patient consent was not required for inclusion. Collection of patient data was approved by the institutional review board (IRB).
Phase 0
Patients included for baseline analysis (Phase 0) were retrospectively identified via query of the electronic medical record (EMR). Patients with a diagnosis of SAH admitted to the Neuro ICU at the quaternary academic medical center from 2013 to 2014 were included. Patients with perimesencephalic SAH or those for whom comfort measures or hospice care were initiated were not included in our study. No mobilization took place while an EVD was in place. PT/occupational therapy (OT) was not consulted until after the drain was removed; an order for “strict bed rest” remained active until that time. No out-of-bed activity was permitted. While on strict bed rest, the head of bed could be adjusted for patient comfort, with the EVD transducer maintained at the level of the tragus. The head of bed for mechanically ventilated patients was maintained at greater than 30° per hospital protocol. There was no maximum restriction on head of bed elevation. All data points were retrospectively, manually abstracted from the EMR.
Phase I
Subjects for Phases I and II were prospectively identified from an IRB-approved cohort of SAH patients admitted to the Neuro ICU between 2014 and 2016. Phase I lasted for 12 months, from 2014 to 2015, and was characterized by use of a PT/OT-driven protocol [13]. Mobilization occurred only during formal PT/OT sessions with continuous presence of both the therapist and the bedside nurse. Activities included sitting at the edge of the bed, standing in place, and marching in place. No independent time out-of-bed occurred in Phase I.
Phase II
Phase II began in January 2016 and lasted for 8 months. It was defined by use of a nurse-driven protocol (Fig. 1). In Phase II, nurses were empowered to independently mobilize patients. Mobilization could occur prior to initial PT/OT evaluation. Additionally, patients were permitted to remain out of bed in a chair with drain clamped for up to 3 hours at a time. The decision was made to keep the drain clamped for the entirety of the mobilization session in order to prevent overdrainage of cerebrospinal fluid (CSF). Nurses assessed patients’ vital signs (including intracranial pressure [ICP]) and neurologic status hourly. If ICP increased (> 20 mmHg) while a patient was in the chair, the nurse could request an order to drain 10–15 mL of CSF each hour and then re-clamp the drain. Intensity and duration of activity and time out of bed was subject to patient preference, tolerance, and nursing workflow. Data points in both Phases I and II were prospectively tracked by clinical staff.
The primary outcome measure was frequency of patient mobilization. Safety outcomes included elevation of ICP, acute onset of headache during mobilization, and acute focal/worsening of neurologic deficits. Secondary outcomes included ICU and hospital length of stay, rates of tracheostomy and ventriculoperitoneal shunt placement, discharge disposition, and ventilator days.
Subjects
All patients received standard of care for aneurysmal SAH according to a previously published local protocol [14], which included aggressive pre-hospital and pre-operative resuscitation, early aneurysm occlusion, observation and supportive care in the ICU with invasive hemodynamic monitoring, and aggressive prevention and treatment of intracranial hypertension and DCI, consistent with published guidelines [15]. During the study duration, there were no changes to the standard treatment protocol at our institution that may have concomitantly attributed to a decrease in length of stay in the study population. SAH was established radiographically by admission computed tomography scan. Patients were characterized by admission Hunt and Hess (HH) grade [16] and modified Fisher (mF) grade [17]. Demographic data, including age and sex, as well as clinical and laboratory data were recorded. Comorbidity data, including hypertension, coronary artery disease, prior stroke, diabetes, and tobacco and alcohol use, were also documented. Aneurysm size and location were documented from 4-vessel catheter cerebral angiography.
Patients’ eligibility for mobilization was evaluated on a daily basis. Patients were excluded from mobilization if they were unable to tolerate at least 30 min of EVD clamping (as assessed during bed rest), if they exhibited sustained intracranial hypertension (ICP > 20 mmHg), or if they were experiencing symptomatic vasospasm. Daily transcranial Doppler (TCD) studies were conducted for each patient; however, elevated TCD velocities in the absence of clinically significant neurologic changes did not exclude patients from mobilization. Relative mobilization exclusion criteria included fluctuating neurologic examination, pulmonary or cardiovascular instability, and patient refusal. Relative exclusion criteria were evaluated and considered on a daily case-by-case basis by the nursing and medical teams. Strict criteria to define pulmonary and cardiovascular instability were not instituted. Rather, clinicians determined individual stability based upon knowledge of each patient’s baseline and physiologic trends throughout his or her ICU stay. Of note, patient inclusion was not dependent upon level of consciousness, HH and mF score, mechanical ventilation status, or the ability to follow commands. Additionally, the standard of practice at our institution is to send SAH patients to neurointerventional radiology within the first 24 h of admission. Subsequent flat bed rest restrictions ensue for six hours post-intervention. Patients were eligible for mobilization after bed rest restrictions were lifted. No additional changes to the standard care of SAH patients took place throughout the study period.
Statistical Analysis
Statistical analyses were performed using JMP Pro statistical software (SAS) version 12. The χ2 test was used to compare categorical variables. The 2-sample t test was used to compare continuous variables. Multiple regression analyses were conducted to examine the relationships between discharge disposition, length of stay, incidence of tracheostomy placement, and ventilator days and multiple potential predictors. The Wilcoxon Rank Sum test was used for data sets that appeared to be not normally distributed. Significant differences between groups were reported at the α level of 0.05.
Results
The majority of patients in all three phases had an aneurysmal SAH. Average age was similar in all groups, although fewer patients in Phase II were classified as high-grade SAH, as designated by HH grade 4 or 5 (Table 1). All patients, except two, were admitted within 48 h of SAH, and all except one underwent EVD placement within the first 48 h of admission. Thirteen patients were excluded from analysis due to death.
No patients in the Phase 0 cohort (n = 15) were mobilized with an EVD. In Phase I (n = 24), first mobilization occurred 14 days earlier (Table 2; hospital day 6 versus hospital day 20; p < 0.0001) with implementation of the therapy-driven protocol. Using the nurse-driven protocol (Phase II; n = 17), mobilization occurred on average 1 day earlier than with the therapy-driven protocol (p = 0.099). Patients were mobilized more frequently with each iteration of our trial, progressing from complete bed rest in Phase 0 to an average of 3 mobilization sessions per ICU stay in Phase I (p < 0.0001) and 7 sessions per ICU stay in Phase II (p < 0.0001). A total of 71 mobilization sessions were completed for the 24 patients in Phase I, all of which were completed jointly with PT/OT and nurses. Mobilization sessions in Phase I focused on transitioning from sitting to standing and transferring from bed to chair. A total of 120 mobilization sessions occurred among the 17 patients in Phase II, which represents a 41% increase in the total number of mobilization sessions. Of these 120, 57 sessions (47.5%) occurred jointly between PT/OT and nurses, and 63 sessions (52.5%) were conducted independently by nurses. The most common activities independently completed by nurses included transferring from bed to chair, walking to the commode, and ambulating in the hallway. When nurses independently mobilized a patient, PT shifted their focus to advanced gait training and stamina (increasing the distance of ambulation). Occupational therapists focused on ADLs, coordination, and communication.
Hospital length of stay progressively decreased with each Phase, although not significantly. ICU length of stay in Phase II trended lower (p = 0.007) when compared with Phase I. In addition, incidence of tracheostomy placement and time on the ventilator lessened over time. No patients in Phase II received a tracheostomy, although severity of injury may have been lower in this cohort. Discharge disposition significantly improved from Phase 0 to Phase II (Table 2).
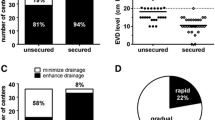
Prior to implementation of a mobilization protocol, 60% of SAH patients with an EVD were discharged to home or acute rehab, while the remainder went to a long-term acute care hospital (LTACH), skilled nursing facility (SNF), or another acute care hospital. In Phase II, all patients were discharged to home or acute rehab. In a multivariate analysis, the odds of discharge to home or rehab versus LTACH or SNF were 3.83 (95% confidence interval, 1.14–9.16) for mobilized patients, independent of age, Glasgow Coma score (GCS) at first mobility session, and high HH grade (Table 3). Mobilization of patients was not significantly independently associated with hospital length of stay, tracheostomy placement, or ventilator days.
Reasons for aborted sessions and premature cessation of mobilization were documented by PT/OT and nursing staff. In Phase I, there were 23 instances where PT/OT consultation was intended but no mobilization actually occurred. Reasons for withholding mobilization included acute worsening of neurologic examination, hyper- or hypotension, and increased ICP. Four sessions in Phase I were aborted mid-session due to pain, increased ICP, and hypotension. In Phase II, there were 11 attempted-but-never-initiated mobilization sessions. Reasons for withholding mobilization included traveling for testing or procedures, hypertension, increased ICP, and symptomatic vasospasm. Only one session was aborted mid-session due to elevated ICP. No falls, incidental medical device dislodgement (central venous catheters, arterial lines, endotracheal tubes, enteric feeding tubes, indwelling urinary catheters), acute hypoxia, new onset arrhythmias, prolonged elevated ICP, or neurologic changes occurred in association with early mobilization throughout the duration of data collection for our quality improvement initiative. Two EVD dislodgements occurred during the study period, although neither occurred during mobilization, nor were these incidents directly related to mobilization. Changes in blood pressure that could be adequately managed with titration of pre-existing continuous vasoactive infusions or changes in heart rate unaccompanied by changes in blood pressure or other clinical findings were not considered to be adverse events.
Discussion
Early mobilization refers to a broad range of activities, and no clear definition of early mobilization has yet been defined in the literature [18]. For our purposes, mobilization was defined as any patient activity that occurred seated at the edge of bed or any out-of-bed physical activity. The ideal timing of early mobilization after admission or injury [18], the recommended frequency of mobilization, and the development of standardized nurse-driven mobilization scales are also yet to be defined [19,20,21]. We sought to create an early mobilization protocol that would be safe, feasible, and result in earlier and more frequent mobilization. We chose to follow additional specific quality metrics that were informed by previously published benefits of early mobilization.
Safety/Complications
Early mobilization in the ICU is met with multiple barriers, including the presence of invasive devices [22]. Invasive neuromonitoring devices may deter early mobilization for various reasons, including fear of catheter dislodgement, hemorrhage, or inappropriate CSF drainage [23]. These drains and monitors have historically required strict bed rest. Strategies to promote mobilization in ICU patients with devices include a stepwise mobilization protocol; securement of lines, drains, and tubes; pre-mobilization preparation; interdisciplinary teamwork; and clear delineation of roles [22].
Although mobilizing patients with invasive devices carries inherent risk, the consequences of prolonged bed rest, including significant muscle wasting and reduced functional status, are profound [23, 24]. Recent literature has demonstrated that early mobilization in the ICU can be a safe and feasible intervention, with few adverse events recorded [7, 13]. Both therapy-driven and nurse-driven protocols have been studied with similar safety outcomes [5, 19, 25,26,27,28,29,30,31,32,33,34].
One major concern with mobilizing patients during the acute phase following SAH is exacerbation of cerebral vasospasm or worsening DCI. Kung et al. [35] demonstrated the safety of changing head of bed position in SAH patients by measuring cerebral blood flow via TCD and thermal diffusion probe. Karic et al. [36] prospectively evaluated the effect of early mobilization on complications during the acute phase and within 90 days after SAH. In the early mobilization group, cerebral vasospasm was less frequent and less severe when compared to the control group. Additionally, the risk of severe vasospasm was reduced by 30% with each progression in level of mobilization achieved during the first 4 days after aneurysm repair. Acute and chronic hydrocephalus, pulmonary infections, thromboembolic events, and death before discharge or within 90 days after the ictus were similar between the intervention and control groups. Olkowski et al. [18] also described an early mobilization program for patients with a SAH as both safe and feasible.
In our quality improvement initiative, every patient in the ICU was evaluated daily by the interdisciplinary team for mobilization readiness. Our goal was to enter PT/OT consultation orders as early as possible after admission. Confirmation of PT/OT consultation order entry was confirmed for each patient every morning. PT/OT and bedside nurses evaluated patients for the presence of any defined mobilization exclusion criteria and analyzed each patient’s current clinical status for the presence of any relative exclusion criteria as defined. Any questionable circumstances related to mobilization readiness were communicated to the medical team for expert guidance. No catheter dislodgements or bleeding complications were attributable to use of our early mobilization protocol. To prevent inappropriate CSF drainage, our protocol required double clamping of the EVD while the patient was out of bed. With an order, nurses could drain a specified amount of CSF each hour.
Nurse-Driven Mobilization Protocols
The primary hypothesis in Phase II of our early mobilization initiative was that implementation of a nurse-driven early mobilization protocol would lead to earlier and more frequent mobilization. In Phase I, patients could be mobilized only during formal PT sessions. With our PT/OT resources and staffing model, a maximum of seven out of our twenty-two patients could be mobilized each day. In Phase II of our initiative, nurses were able to independently mobilize their patients and do so prior to PT/OT consultation. With implementation of a nurse-driven protocol, the time from admission to first mobilization decreased, although not significantly, from 6.0 days in Phase I to 4.9 days in Phase II (p = 0.15). However, the number of mobility sessions per patient significantly increased from 3.0 to 7.1 (p < 0.0001). This represents an increase of 41% in the number of mobilization sessions and a 230% increase in the number of mobilization sessions per patient from Phase I. Moreover, half of the 120 mobilization sessions that occurred in Phase II were conducted independently by nurses. This corroborates findings from previous studies that have measured mobilization rates in critically ill patients [37,38,39]. We found that our patients who were mobilized using a nurse-driven protocol were mobilized more frequently, although not earlier.
Length of Stay
Early mobilization in the ICU also has implications for decreased ICU and hospital length of stay. Winkelman et al. [21] demonstrated a significant reduction in ICU length of stay among medical and surgical ICU patients who were mobilized with the use of an early mobilization protocol. Titsworth et al. [20] demonstrated a similar finding among Neuro ICU patients with use of the Progressive Upright Mobility Protocol Plus.
Patients with SAH at our hospital are treated by a standard pathway. Comparing no mobilization (Phase 0) to nurse-driven mobilization (Phase II), average ICU length of stay decreased by nearly 5 days (p = 0.078). Although ICU length of stay was not significantly reduced, overall hospital length of stay among Phase II patients was, on average, 1 week shorter (p = 0.031) when compared to patients who were not mobilized.
Ventilator Days and Tracheostomy Placement
Early mobilization in the ICU may also improve respiratory muscle strength, resulting in earlier liberation from the ventilator or decreased incidence of tracheostomy placement [9, 10]. Patients who were mobilized with nurses (Phase II) received fewer days of mechanical ventilation than patients who were not mobilized (p = 0.013). In addition, among patients who received no mobilization (Phase 0), 40% received a tracheostomy. No patients in Phase II who were mobilized with the nurse-driven protocol received a tracheostomy (p = 0.004). In light of these results, it is important to note that fewer patients in Phase II were intubated (30% versus 26.7% of patients in Phase 0 and 25% of patients in Phase I).
Discharge Disposition
Early mobilization is associated with improved muscle strength and function [7], improved peripheral muscle strength, and greater functional independence [9, 10]. Because of these data, we hypothesized that implementation of an early mobilization protocol in the Neuro ICU would better prepare patients for longer duration of therapy that is required to qualify for acute rehabilitation services. This would result in better discharge disposition. We found that fewer patients who were mobilized with a therapy-driven protocol were discharged to an LTACH or SNF. However, no patients mobilized with a nurse-driven protocol were discharged to LTACH or SNF. All patients were discharged home or to acute rehab. The Phase II cohort was slightly smaller than the other two cohorts, and had several patients with lower HH/mF scores. Moreover, patients in Phase II were younger, on average, than those in the other cohorts. ICU and hospital LOS were shorter in this cohort as well. It may be that these patients were generally less critically ill, which thus may account for the improved outcomes. However, multivariate analysis revealed better discharge disposition independent of severity of illness, as measured by initial HH, mF, or GCS scores. It should be pointed out that the effect of high-grade HH score was not associated with discharge disposition, which runs counter to much of the established literature. This is likely due to the few high-grade patients that were included in this study. We suspect that most patients with high HH scores transitioned to hospice and were thus not eligible for this study.
Between Phase 0 and Phase I, there was statistically significant improvement between the combined discharge disposition.
Limitations
These findings are met with several limitations. This initiative represents a quality improvement initiative in a single Neuro ICU. We restricted the analysis to a specific patient population to limit the variability, but in so doing, the sample size remained small, and power was lacking to establish a veritable effect on outcomes. Moreover, there was decreased acuity of SAH patients during Phase II as well as a trend toward fewer female patients in the cohort. Such variability may have considerable impact on nearly all outcome measures. Data regarding the duration of mobilization sessions, employed mobilization maneuvers, and functional milestones were recorded inconsistently. Therefore, qualitative differences in mobilization sessions may not be accounted for. Additionally, the daily PT/OT prioritization process was not standardized during the study. Patient rapport and disposition needs of all other Neuro ICU patients likely impacted on daily evaluation who was prioritized for mobilization. It should also be made clear that during Phase II, although mobilization was nurse-driven, PT/OT remained a vital and integral part of the mobilization protocol. Lastly, data collection for Phase 0 occurred retrospectively.
Future Research Opportunities
Many studies in general medical/surgical ICUs exclude or limit participation in patients with acute neurologic injury or patients who are not able to volitionally participate in activities [5, 11, 21, 30, 31, 37]. The study of early mobilization in patients with acute neurologic injury warrants additional study among both the overall neurocritical care population and specific subpopulations and disease states. There is also opportunity to study the neuroscience population and effects of early mobilization on quality metrics such as incidence of DVT, skin ulceration, delirium, etc. Study of the types of mobilization interventions with the greatest safety profile, feasibility, and impact on outcome would aid in determining the types of resources and protocols most appropriate for the Neuro ICU. Additionally, guidelines for management of patients with SAH do not address ideal timing or safety of mobilizing patients after aneurysm treatment [40, 41]. The creation and testing of formal mobilization protocols for use in the Neuro ICU is also needed. Lastly, by relieving the case load of routine mobilization, a nurse-driven mobilization approach may permit PT/OT to perform novel and advanced therapy techniques, such as vented ambulation, cycle ergometry and advanced gait training for the most highly complex patients in the ICU.
Conclusions
We conclude that nurse-driven mobilization in SAH patients with EVDs is both safe and feasible. Allowing nurses to drive mobilization leads to more frequent ambulation than when patients are mobilized only during formal PT/OT sessions. Nurse-driven mobilization may be associated with improved discharge disposition, although the small sample size precludes the establishment of causation. Additionally, a nurse-driven mobilization protocol may allow for PT/OT to devote more time to novel or advanced therapy techniques for the most highly complex patients in the ICU. While these preliminary data are encouraging, further study is warranted regarding mobilization of SAH patients, patients with EVDs, and nurse-driven mobilization protocols.
References
Parry SM, Puthucheary ZA. The impact of extended bed rest on the musculoskeletal system in the critical care environment. Extrem Physiol Med. 2015;4:16.
Brummel NE, Balas MC, Morandi A, Ferrante LE, Gill TM, Ely EW. Understanding and reducing disability in older adults following critical illness. Crit Care Med. 2015;2015(43):1265–75.
Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomized controlled trial. Lancet. 2009;373:1874–82.
Brahmbhatt N, Murugan R, Milbrandt EB. Early mobilization improves functional outcomes in critically ill patients. Crit Care. 2010;14:321.
Mulkey M, Bena JF, Albert NM. Clinical outcomes of patient mobility in a neuroscience intensive care unit. J Neurosci Nurs. 2014;46:153–61.
Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–600.
Tipping CJ, Harrold M, Holland A, Romero L, Nisbet T, Hodgson CL. The effects of active mobilization and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med. 2017;43:171–83.
Iwashyna TJ. Trajectories of recovery and dysfunction after acute illness, with implications for clinical trial design. Am J Respir Crit Care Med. 2012;186(4):302–4.
Kayambu G, Boots R, Paratz J. Physical therapy for the critically ill in the ICU: a systematic review and meta-analysis. Crit Care Med. 2013;41:1543–54.
Miller MA, Govindan S, Watson SR, Hyzy RC, Iwashyna TJ. ABCDE, but in that order? A cross-sectional survey of Michigan intensive care unit sedation, delirium, and early mobility practices. Ann Am Thorac Soc. 2015;12:1066–71.
Schaller SJ, Anstey M, Blobner M, et al. Early, goal-directed mobilisation in the surgical intensive care unit: a randomized controlled trial. Lancet. 2016;388:1377–88.
Olkowski BF, Shah SO. Early mobilization in the neuro-ICU: How far can we go? Neurocrit Care. 2017;27:141–50.
Moyer M, Young B, Maloney-Wilensky E, et al. Implementation of an early mobility pathway in neurointensive care unit patients with external ventricular devices. J Neurosci Nurs. 2017;49:102–7.
Sanborn MR, Thom SR, Bohman LE, Stein SC, Levine JM, Milavanova T, et al. Temporal dynamics of microparticle elevation following subarachnoid hemorrhage. J Neurosurg. 2012;117:579–86.
Bederson JB, Connolly ES Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the stroke council. Am Heart Assoc Stroke. 2009;40:994–1025.
Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28(1):14–20.
Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES Jr, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery. 2006;59(1):21–7.
McWilliams DJ, Atkins GA, Boyers M, Lea T, Hodson J, Snelson C. Feasibility and reliability of the manchester mobility score as a measure of physical function within the intensive care unit. ACPRC J. 2016;48:26–33.
Olkowski BF, Devine MA, Slotnick LE, et al. Safety and feasibility of an early mobilization program for patients with aneurysmal subarachnoid hemorrhage. Phys Ther. 2015;93:208–15.
Titsworth WL, Hester J, Correia T, et al. The effect of increased mobility on morbidity in the Neurointensive care unit. J Neurosurg. 2012;116:1379–88.
Winkelman C, Johnson KD, Hejal R, et al. Examining the positive effects of exercise in intubated adults in ICU: a prospective repeated measures clinical study. Intensive Crit Care Nurs. 2012;28:307–18.
Dubb R, Nydahl P, Hermes C, et al. Barriers and strategies for early mobilization of patients in intensive care units. Ann Am Thorac Soc. 2016;13:724–30.
Kocan MJ, Lietz H. Special considerations for mobilizing patients in the neurointensive care unit. Crit Care Nurs Q. 2013;36:50–5.
Griffiths RD, Hall JB. Intensive care unit-acquired weakness. Crit Care Med. 2010;38:779–87.
Chavez J, Johnson Bortolotto S, Paulson M, Huntley N, Sullivan B, Babu A. Promotion of progressive mobility activities with ventricular assist and extracorporeal membrane oxygenation devices in a cardiothoracic intensive care unit. Dimens Crit Care Nurs. 2015;34:348–55.
Clark DE, Lowman JD, Griffin RL, Matthews HM, Reiff DA. Effectiveness of an early mobilization protocol in a trauma and burns intensive care unit: a retrospective cohort study. Phys Ther. 2013;93:186–96.
Bailey P, Thomsen GE, Spuhler VJ, Blair R, et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–45.
Burtin C, Clerckx B, Robbeets C, et al. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009;37:2499–505.
Morris PE, Goad A, Thompson G, et al. Early intensive care unit mobility in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–43.
Needham DM, Korupolu R. Rehabilitation quality improvement in an intensive care unit setting: implementation of a quality improvement model. Top Stroke Rehabil. 2010;17:271–81.
Pohlman MC, Schweickert WD, Pohlman AS, et al. Feasibility of physical and occupational therapy beginning from initiation of mechanical ventilation. Crit Care Med. 2010;31:2089–94.
Stiller KA, Phillips AC, Lambert P. The safety of mobilisation and its effect on hemodynamic and respiratory status of intensive care patients. Physiother Theory Pract. 2004;20:175–85.
Thomsen C, Goad A, Taylor K, et al. The role of the critical care nurse in the organization and management of an early ICU mobility team. Poster presented at the National Teaching Institute; 2–8 May 2008; Chicago, IL.
Zanni JM, Korpolu R, Fan E, et al. Rehabilitation therapy and outcomes in acute respiratory failure: an observational pilot. J Crit Care. 2009;25:254–62.
Kung DK, Chalouhi N, Jabbour PM et al. Cerebral blood flow dynamics and head-of-bed changes in the setting of subarachnoid hemorrhage. BioMed Res Int. 2013;2013:640638. https://doi.org/10.1155/2013/640638.
Karic T, Roe C, Nordenmark TH, Becker F, Sorteberg W, Sorteberg A. Effect of early mobilization and rehabilitation on complications in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2017;126:518–26.
Drolet A, DeJuilio P, Harkless S, et al. Move to improve: the feasibility of using an early mobility protocol to increase ambulation in the intensive and intermediate care settings. Phys Ther. 2013;93:197–207.
Harris CL, Shahid S. Physical therapy-driven quality improvement to promote early mobility in the intensive care unit. Proc (Bayl Univ Med Cent). 2014;27:203–7.
Hodgson CL, Bailey M, Bellomo R, et al. A binational multicenter pilot feasibility randomized controlled trial of early goal-directed mobilization in the ICU. Crit Care Med. 2016;44:1145–52.
Diringer MN, Bleck TP, Hemphill JC, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care. 2011;15:211–40.
Connolly ES, Rabinstein AA, Carhuapona JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43:1711–37.
Funding
None.
Author information
Authors and Affiliations
Contributions
BY is responsible for writing, editing, collecting feedback, and submitting the article. She served as a clinical consultant for ArjoHuntleigh during portions of the study. MM participated in the protocol creation, literature review, and review and editing of the article. WP participated in the protocol creation and review and editing of the article. DK participated in review and editing of the article. EZ participated in review and editing of the article. MK participated in statistical analysis, review, and editing of the article.
Corresponding author
Ethics declarations
Conflicts of Interest
All authors declare that they have no conflicts of interest.
Ethical Approval
This study was approved by the institutional review board (University of Pennsylvania; IRB 825053).
Informed Consent
Regarding the retrospective portion of this project, for this type of study formal consent is not required. Regarding the prospective quality improvement portion of this project, it was approved by the University of Pennsylvania’s IRB as a quality improvement project and thus did not require formal consent of patients involved.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Young, B., Moyer, M., Pino, W. et al. Safety and Feasibility of Early Mobilization in Patients with Subarachnoid Hemorrhage and External Ventricular Drain. Neurocrit Care 31, 88–96 (2019). https://doi.org/10.1007/s12028-019-00670-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-019-00670-2