Abstract
Background
Infections are a common medical complication in hemorrhagic stroke patients, with vancomycin commonly used as empiric therapy. The purpose of this study was to evaluate the pharmacokinetic parameters of vancomycin in hemorrhagic stroke patients.
Methods
This was a retrospective study of adult patients with aneurysmal subarachnoid hemorrhage (aSAH) or intracerebral hemorrhage (ICH) admitted between May 2010 and February 2015 who received vancomycin. Predicted pharmacokinetic parameters based on population data were compared with calculated pharmacokinetic parameters based on serum trough concentrations.
Results
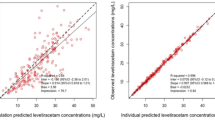
Eighty aSAH patients and 66 ICH patients met inclusion criteria. In the aSAH group, the mean dosing regimen was 17.6 ± 4 mg/kg every 12 (8–12) h. The mean measured trough concentration was lower than the predicted trough concentration (9.9 ± 4.1 vs. 19 ± 8.7 μg/mL; p < 0.001). The mean calculated elimination rate constant was higher than the predicted value (0.135 ± 0.04 vs. 0.092 ± 0.03 h−1; p < 0.001), and the mean calculated half-life was lower than predicted (5.7 ± 1.8 vs. 8.3 ± 2.9 h; p < 0.001). In the ICH group, the mean dosing regimen was 15.9 ± 4.3 mg/kg every 12 (8–12) h. Similarly, the mean measured trough concentration was lower than the predicted trough concentration (10.7 ± 4.6 vs. 17.5 ± 8.5 μg/mL; p < 0.001). The mean calculated elimination rate constant was higher than the predicted value (0.106 ± 0.03 vs. 0.079 ± 0.02 h−1; p < 0.001), and the mean calculated half-life was lower than predicted (7.2 ± 2.3 vs. 9.6 ± 3.2 h; p < 0.001).
Conclusions
Patients with hemorrhagic stroke exhibited pharmacokinetic alterations favoring increased elimination of vancomycin when compared to predicted pharmacokinetic parameters based on population data. This may result in underexposure to vancomycin, leading to treatment failure and other medical complications.

Similar content being viewed by others
References
Claassen J, Vu A, Kreiter KT, et al. Effect of acute physiologic derangements on outcome after subarachnoid hemorrhage. Crit Care Med. 2004;32:832–8.
Wartenberg KE, Mayer SA. Medical complications after subarachnoid hemorrhage. Neurosurg Clin N Am. 2010;21:325–38.
Hemphill JC III, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032–60.
Wartenberg KE, Mayer SA. Medical complications after subarachnoid hemorrhage: new strategies for prevention and management. Curr Opin Crit Care. 2006;12(2):78–84.
Wartenberg KE, Schmidt JM, Claassen J, et al. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;34:617–23.
Naidech AM, Bendok BR, Tamul P, et al. Medical complications drive length of stay after brain hemorrhage: a cohort study. Neurocrit Care. 2009;10:11–9.
Udy AA, Baptista JP, Lim NL, et al. Augmented renal clearance in the ICU: results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations. Crit Care Med. 2014;42:520–7.
Udy AA, Roberts JA, Lipman J. Implications of augmented renal clearance in critically ill patients. Nat Rev Nephrol. 2011;7:539–43.
Balami JS, Buchan AM. Complications of intracerebral haemorrhage. Lancet Neurol. 2012;11:101–18.
Liotta EM, Singh M, Kosteva AR, et al. Predictors of 30-day readmission after intracerebral hemorrhage: a single-center approach for identifying potentially modifiable associations with readmission. Crit Care Med. 2013;41:2762–9.
Singh M, Guth JC, Liotta E, et al. Predictors of 30-day readmission after subarachnoid hemorrhage. Neurocrit Care. 2013;19:306–10.
Ohwaki K, Yano E, Nagashima H, Nakagomi T, Tamura A. Impact of infection on length of intensive care unit stay after intracerebral hemorrhage. Neurocrit Care. 2008;8:271–5.
Cook AM, Arora S, Davis J, Pittman T. Augmented renal clearance of vancomycin and levetiracetam in a traumatic brain injury patient. Neurocrit Care. 2013;19:210–4.
Morbitzer KA, Jordan JD, Rhoney DH. Vancomycin pharmacokinetic parameters in patients with acute brain injury undergoing controlled normothermia, therapeutic hypothermia, or pentobarbital infusion. Neurocrit Care. 2015;22:258–64.
Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43:1711–37.
Frontera JA, Fernandez A, Schmidt JM, et al. Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke. 2009;40:1963–8.
Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984;25:433–7.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
Wurtz R, Itokazu G, Rodvold K. Antimicrobial dosing in obese patients. Clin Infect Dis. 1997;25:112–8.
Sawyer WT, Canaday BR, Poe TE, et al. Variables affecting creatinine clearance prediction. Am J Hosp Pharm. 1983;40:2175–80.
Ambrose PJ, Winter ME. Basic clinical pharmacokinetics. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2004. p. 451–76.
Udy A, Boots R, Senthuran S, et al. Augmented creatinine clearance in traumatic brain injury. Anesth Analg. 2010;111:1505–10.
May CC, Arora S, Parli SE, Fraser JF, Bastin MT, Cook AM. Augmented renal clearance in patients with subarachnoid hemorrhage. Neurocrit Care. 2015;23(3):374–9.
Lin Wu FL, Liu SS, Yang TY, et al. A larger dose of vancomycin is required in adult neurosurgical intensive care unit patients due to augmented clearance. Ther Drug Monit. 2015;37(5):609–18.
Campassi ML, Gonzalez MC, Masevicius FD, et al. Augmented renal clearance in critically ill patients: incidence, associated factors and effects on vancomycin treatment. Rev Bras Ter Intensiva. 2014;26:13–20.
Baptista JP, Sousa E, Martins PJ, Pimentel JM. Augmented renal clearance in septic patients and implications for vancomycin optimisation. Int J Antimicrob Agents. 2012;39:420–3.
Udy AA, Roberts JA, Boots RJ, Paterson DL, Lipman J. Augmented renal clearance: implications for antibacterial dosing in the critically ill. Clin Pharmacokinet. 2010;49:1–16.
Andaluz N, Zuccarello M. Recent trends in the treatment of spontaneous intracerebral hemorrhage: analysis of a nationwide inpatient database. J Neurosurg. 2009;110:403–10.
McQueen EG, Morrison RB. The effects of synthetic angiotensin and noradrenaline on blood pressure and renal function. Br Heart J. 1961;23(1):1–6.
Dorsch NW, King MT. A review of cerebral vasospasm in aneurysmal subarachnoid haemorrhage Part I: incidence and effects. J Clin Neurosci. 1994;1:19–26.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The IRB at the University of North Carolina at Chapel Hill granted a waiver of informed consent for the study procedures.
Rights and permissions
About this article
Cite this article
Morbitzer, K.A., Jordan, J.D., Sullivan, K.A. et al. Vancomycin Pharmacokinetic Parameters in Patients with Hemorrhagic Stroke. Neurocrit Care 25, 250–257 (2016). https://doi.org/10.1007/s12028-016-0264-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-016-0264-8