Abstract
Background
Cerebral autoregulation is increasingly recognized as a factor that requires evaluation when managing poor grade aneurysmal subarachnoïdal hemorrhage (aSAH) patients. In this single center pilot study, we investigated whether intraventricular intracranial pressure (ICP) derived when extraventricular drain (EVD) is open can be used to calculate dynamic autoregulation estimates in ICU aSAH patients.
Methods
Ten patients with the diagnosis of aSAH as confirmed by computed tomography (CT) and CT-angiography were enrolled. ICP was monitored via a transducer connected to the most proximal side exit of the EVD catheter. From at least 30 min periods of brain monitoring before, during, and after temporarily EVD closure, commonly used indexes of dynamic cerebral autoregulation were calculated.
Results
Preserved pulsatile ICP signals were seen with open EVD. There were no significant changes in parameters describing cerebral autoregulation between EVD open and closed conditions. Power spectra of ABP and ICP showed no significant changes for the selected frequency ranges. There was a small significant increase in absolute ICP [2.4 (3.8) mmHg, p < 0.001] upon short-term EVD closure. Cerebral spinal reserve capacity (RAP index) worsened significantly by short-term EVD closure.
Conclusions
Due to preserved slow fluctuations in the ICP signal, an open EVD system can be used to calculate dynamic autoregulation indices in aSAH patients requiring intensive care monitoring with the pressure measurement from the most proximal part of drain. If these results are confirmed in larger study, this technique can open the way for investigating the role of autoregulation disturbance in aSAH patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately, 70 % of patients who experience aneurysmal subarachnoid hemorrhage (aSAH) survive. Early resuscitation and obliteration of the aneurysm can prevent the catastrophe of a rebleed, but approximately 25 % of patients with aSAH deteriorate over the following days, necessitating intensive care (IC) admission [1]. Secondary deterioration may be due to cerebral vasospasm, acute hydrocephalus, electrolyte disturbances, or delayed cerebral ischemia (DCI). External ventricular drain (EVD) placement is standard of care in the management of aSAH-associated hydrocephalus.
Positron emission tomography (PET) imaging in patients with aSAH reveals that in parallel to cerebral vasospasm various hemodynamic abnormalities ranging from hypoperfusion to hyperemia can be observed [2]. Furthermore, hypoperfusion and oligemia can frequently occur without vasospasm [3]. These findings collectively suggest that mechanisms other than vasospasm should be considered when investigating the underlying causes of delayed deterioration. Early brain injury resulting from the initial bleeding, microvascular spasm, microthrombosis, spread of cortical depolarizations, and failure of cerebral blood flow autoregulation (CA) are processes that have all been implicated in delayed deterioration following aSAH [4].
Like in traumatic brain injury, CA is increasingly recognized as a factor that requires evaluation when managing poor grade aSAH patients [5]. Recent prospective studies demonstrate that indirect indices of CA can be used to predict long-term outcome after aSAH [6–8]. Importantly, these indices become abnormal in patients before onset of radiographically identifiable arterial narrowing and in those who do not develop detectable vasospasm [9, 10]. These measurements can be done non-invasively with NIRS or TCD or in neuro IC with insertion of additional intracerebral devices, like parenchymal intracranial pressure (ICP) and/or partial brain tissue oxygen probes. Theoretically, the EVD-derived intraventricular ICP signal can also be used to calculate CA estimates. However, because resistance to CSF flow may generate an important pressure gradient in the drain and attenuate dynamics of measured slow waves of ICP, this attractive method is rarely used in aSAH patients [10]. Although their data showed that only with nearly complete blockage of all draining EVD holes unreliable absolute ICP measurements will occur, Birch et al. advised temporarily closure of the drain for intermittent monitoring [11]. Closure of the EVD, however, interferes with hydrocephalus treatment.
Therefore, we investigated whether intraventricular ICP derived when EVD is open can be used to calculate CA estimates in aSAH patients managed in IC unit. In this prospective pilot study, we compared 30 min periods of brain monitoring before, during, and after temporarily EVD closure.
Methods and Materials
Patients
This observational study was performed with approval of the local ethics committee in the University Medical Center of Groningen. Ten patients with the diagnosis of aSAH as confirmed by computed tomography (CT) and CT-angiography were enrolled into this prospective study from August 2013 until April 2014 (7 months). Collected data included age, sex, and initial neurological status according to the World Federation of Neurosurgical Societies (WFNS), location of aneurysm, EVD reservoir height, medication, and treatment intervention (surgical clipping or endovascular coiling). In our center, around 25 aSAH patients with EVD are admitted every year for monitoring and IC treatment.
Patients received neurointensive care treatment according to a standard local protocol aiming at homeostasis in terms of fluid balance, mean arterial blood pressure (MABP), serum glucose, and arterial blood gases. If necessary, patients were sedated and ventilated with Propofol and Morphine, and received hypertensive therapy in case of suspected vasospasm [12]. Nimodipine 6 × 60 mg was administered through a nasogastric tube in all patients. None of the patients received statins or erythropoietin during IC unit stay. All patients had a proven aneurysmal bleed (CTA or DSA) and an EVD with initial height of the reservoir levelled at 10 cm above Monro. Exclusion study criteria were profound restlessness, hemodynamic instability, signs of infection (systemic or ventriculitis/meningitis), or marked hydrocephalus preventing closure of the EVD for half an hour.
Measurements
For this study, ICP was monitored via a transducer (Baxter, Healthcare Corp. Cardiovascular Group, Irvine, CA) connected to the most proximal side exit of the EVD catheter (10–15 cm distance to the skull). Transducer was referenced to the Foramen of Monro. Invasive MABP was obtained by an arterial line placed in the radial artery with transducer referenced to right atrial level. Data were stored on a laptop computer with a sample frequency of 200 Hz for later offline analysis with ICM + software (http://www.neurosurg.cam.ac.uk/pages/ICM). Measurements were performed preferably the first week after hemorrhage and done by the same researcher (MJHA). Cerebral perfusion pressure (CPP) was calculated as the difference between MABP and ICP. Time averaged means for MABP, ICP, CPP, heart rate (HR), and if available, the ventilation parameter end-tidal CO2 was calculated and stored every 60 s.
Protocol
Three conditions were measured in a fixed order with time blocks of more than 30 min each. During the first condition, the EVD drain was open (condition 1: ‘open before’), thereafter closed (condition 2: ‘closed’), and reopened (condition 3: ‘open after’). During the measurements, nursing activities were limited, and potential artificial periods were marked in the software by the researchers.
Signal Processing and Analysis
The recorded signals were visually inspected for artifacts. Artifacts of short duration were removed by linear interpolation. Further, processing and calculation of the CA indices were performed in LabVIEW (LabVIEW 2012, National Instruments, Austin, Texas, USA) or ICM + software.
Indices of Cerebrovascular Pressure Reactivity (PRx/PAx)
The PRx index was calculated as the moving linear (Pearson) correlation coefficient between 10 s values of MABP and ICP from the previous 300 s of monitoring with 80 % overlap, that is, every 60 s a new PRx value was calculated. PRx has been previously validated to allow for a continuous assessment of cerebrovascular pressure reactivity as a key component of CA at the patient’s bedside and is described in detail elsewhere [13]. In brief, ICP is a surrogate of cerebral blood volume and flow. PRx quantifies the relationship between spontaneous fluctuations of MABP and ICP and thus quantifies the cerebrovascular autoregulatory status. PRx may vary on a scale between −1 and +1 and displays impaired CA when ICP passively follows MABP, meaning a good correlation between the 2 parameters exists and PRx is high. When pressure reactivity is intact, ICP is relatively unaffected by changes in MABP so that no correlation between MABP and ICP can be observed and PRx is near 0 or negative. Similar to PRx, the relationship between slow MABP fluctuations and the ICP pulse amplitude (AMP) was calculated using a moving correlation technique to reflect the state of cerebral vasoreactivity (PAx index) potentially more robust at lower ICP values [14].
Transfer Function Analyses
Preprocessing of ICP and MABP signals includes low-pass filtering (Butterworth, zero shift, 1 Hz cut-off frequency), median filtering (500 samples), and resampling to 10 Hz. Zero mean signals were created, and detrending was performed. TFA was executed using averaging with Welch’s method (2,000 samples, 50 % overlap, Hanning window). Subsequently, coherence, phase shift, and power spectral density (PSD: i.e., power normalized by frequency bin width) were calculated using LabVIEW functions. The data were exported in 20 bins of 0.005 Hz in the range from 0.005 to 0.1 Hz. This range is chosen because it overlaps the typical range of spontaneous slow MABP waves. We divided the results in a very low frequency (VLF) range (0.005–0.05 Hz), a low frequency (LF) range (0.02–0.065 Hz), and a high frequency (HF) range (0.055–0.1 Hz). The LF range is thought to be the optimal cerebrovascular pressure reactivity frequency range [15]. The coherence significance 95 % confidence interval (95 % CI) limit was calculated using the following formula: \( 1 - (0.05)^{{\frac{1}{{{\text{L}} - 1}}}}, \) where L is the number of data segments. Estimated coherence values below this line can be seen as evidence for a lack of linear association between MABP and ICP [16]. Therefore, phase shifts between ICP and MABP were not analyzed for frequencies with coherence values lower than the calculated 95 % CI limit. Mean values of coherence and phase were also calculated in the 3 frequency ranges by averaging the values from these bins. The total PSD of MABP and ICP was calculated by integration of the power in the 3 frequency ranges.
Amplitude of ICP (AMP) and Index of Cerebrospinal Reserve (RAP)
AMP was calculated as the AMP of the fundamental harmonic of the Fourier decomposition of the ICP pulse waveform from consecutive, 10-s time periods. Measuring the fundamental harmonics instead of the full shape of the AMP implies some loss of information. Generally, good correlation between the AMP first harmonic and the peak-to-peak AMP indicates poor cerebrospinal compensatory reserve [17]. The RAP index was calculated as a Pearson correlation coefficient between mean ICP and AMP from a 5-min period (amounting to 30 data points) with update every 60 s [18].
Statistical Analysis
Data are presented as mean ± standard deviation (SD). No formal power calculation was performed. Paired t tests were used for the following comparisons: open before versus closed, closed versus open after, and open before versus open after. Normality of variable distribution was evaluated with the Shapiro–Wilk statistic, and if necessary logarithmic transformation was used. Statistical significance was set at a p value of <0.05. All statistical analyses were executed using statistical software SPSS (SPSS, Inc., Chicago, IL, USA).
Results
Subjects
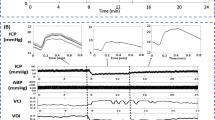
Baseline characteristics of the ten aSAH patients are presented in Table 1. Six measurements were excluded because of short time blocks (<30 min) or due to multiple artifacts. Seven patients had multiple open/close EVD recordings, which resulted in a total of 22 measurements. The group consisted of seven female patients, and the average age was 58 (SD 13) years old. Sixty-four percent of the measurements were done within 3 days after the bleeding ictus, with 4 patients being on the ventilator. Figure 1 shows an example of the time course of ICP and MABP during closure of the EVD. With open drain, all patients had clear visible pulsatile signals during the proximal EVD recordings (Fig. 1a, b), in contrast to recordings from more distal EVD recordings in the past (Fig. 1c) [10].
Example of ICP course during extraventricular drain closure. Time course of intracranial pressure (ICP) in a aSAH patient (female, 57 years old, unsecured ACoM aneurysm) as measured using the proximal part of the extraventricular drain (EVD) (a, b). No major differences are seen looking at the raw pulsatile ICP signal between open (left side) and closed (right side) EVD conditions (b). Time course of ICP in a historical aSAH patient [female, 38 years old, secured MCA aneurysm (clipped)] as measured using the distal part of the EVD and an intraparenchymal transducer, respectively. While the EVD is open (left side), pulsatility of the ICP signal is lost. However, when closing the EVD (right side), the distal EVD-derived and intraparenchymal ICP signal are almost identical [10]. ABP arterial blood pressure, ICP intracranial pressure
Absolute Values During EVD Closure
Both ICP and AMP increased significantly at the end of EVD closure time [ICP: 2.4 (3.8) mmHg, p < 0.001; AMP: 0.35 (0.7) mmHg, p < 0.001] with a subsequent small decrease in CPP [3.0 (7.7) mmHg, p < 0.001] (Table 2).
Spontaneous Oscillations of ICP During EVD Closure
No significant differences were found between PSDs of ICP and MABP before, during, and after EVD closure, indicating preservation of spontaneous oscillations in the predefined frequency ranges (Table 3).
Autoregulation Estimates During EVD Closure
Table 4 presents the results of the CA estimates PRx, PAx, coherence, and phase for the three conditions and separated for each predefined frequency band (Table 4). Only a significant increased HF coherence was found with EVD closure (p < 0.05). The RAP index increased significantly with EVD closure (p < 0.05), indicating poorer pressure–volume compensatory reserve in this situation. Two patients showed ICP increasing >20 mmHg with EVD closure (Fig. 2). In one patient, clearly more positive PAx values were observed abruptly indicating worse CA and likely CSF drainage dependency at this stage (Fig. 2b).
Two examples of ICP recordings with proximal EVD closure and signs of intracranial hypertension. Time course of intracranial pressure (ICP), mean arterial blood pressure (MABP), and derived signals in two aSAH patients with ICP >20 mm Hg during extraventricular drain (EVD) closure. In the first patient (female, 64 years old, unsecured basilar top aneurysm), ICP peaks above 30 mm Hg with mostly low values for PRx and PAx and positive values for RAP, comparable between drain open and closed conditions (a). In the second patient [female, 44 years old, secured ACoM aneurysm (clipped)], ICP peaks above 30 mmHg and more positive PAx and RAP values with drain closure. These changes might suggest more impaired autoregulation and cerebrovascular reserve with drain close and therefore EVD dependency (b). ICP intracranial pressure, CPP cerebral perfusion pressure, AMP amplitude of ICP, PRx pressure reactivity index, PAx vascular reactivity index, RAP cerebrospinal reserve index
Discussion
In this study, we showed that there are no major changes in parameters describing CA between EVD open and closed in aSAH patients admitted to ICU. Power spectra of MABP and ICP also showed no significant changes for the selected frequency ranges as an important perquisite for reliable CA calculation. As expected, there was a small increase in absolute ICP and its AMP upon short-term EVD closure. Cerebral spinal reserve capacity (as presented by the RAP index) worsened significantly by short-term EVD closure.
Autoregulation Indices
In contrast to our expectations, no significant differences in coherence, phase shift, PRx, and PAx between the conditions were found. It was hypothesized that with open EVD, continuous CSF drainage might function as a high pass filter that alters the results of DCA calculation. Possibly, opening the drain will only filter out frequencies very close to DC and will only alter DC values themselves. These changes do not interfere with CA calculation.
ICP and MABP
The increase of absolute ICP (and subsequent CPP decrease) with EVD closure was as expected in our patients who were likely to have decreased absorption of CSF. No direct relation between MABP increase and EVD closure was observed, but an increase in ICP may provoke a raise in MABP to maintain CPP and satisfy the cerebral perfusion demands, especially when autoregulation becomes exhausted and cerebrospinal reserve is lowered [19]. In our cohort, only two patients showed ICP >20 mmHg with short-term EVD closure (Fig. 2).
The increase of absolute ICP with EVD closure is in agreement with the results of a laboratory experiment in the eighties where they tested a hydrodynamic model to simulate intraventricular and parenchymal ICP measurements during different ventricular CSF inflow and outflow levels at high and low absolute ICP levels [20]. Interestingly, they found a large drop in intraventricular ICP with EVD opening and sudden ‘ventricular collapse’ after 2 h with undetectable intraventricular ICP values with the reservoir at midbrain level. In clinical practice, this situation is overcome by setting the EVD reservoir at least at 10 cm above Monro. Unfortunately, the authors did not comment on other ICP characteristics, and the exact location of intraventricular transducer (close or far away from reservoir?) was not reported.
Birch et al. showed that with nearly blockage of all EVD holes by ventricular walls or intraventricular debris, a significant pressure gradient between the inside and outside of the drain may be established with false low ICP readings. Their simple advice is to check ICP values before and after EVD closure to check this avoidable artifact [11]. In our cohort, sudden changes in ICP were not observed making ‘nearly blockage’ of EVD holes less likely.
We showed that slow waves in the autoregulation frequency range are preserved with short-term CSF drainage interruption and can be used for intermittent CA calculation in the early phase of aSAH. This will give further insight in cerebral physiology during aSAH and allow us to study complications like spasms and DCI during follow-up measurements. Furthermore, it offers opportunities for autoregulation monitoring in broad targeting of cerebral perfusion management and prognostification in poor grade aSAH. Whether intermittent CSF drainage when ICP reaches a pre-defined threshold (‘monitor-first’) or continuous CSF drainage (‘drain-first’) at set pressure thresholds is preferred is still a matter of debate [21]. For long-term monitoring (e.g., to introduce, for example, autoregulation-guided treatment (like CPPopt)), EVD pressure readings are not ideal due to unavailability of continuous height correction with patient moving after zeroing. This might be overcome by pressure transducers mounted outside the EVD or using parenchymal probes [11, 22].
Limitations
The study was performed in a selected group of ten aSAH patients limiting statistical power and generalizability of the results. However, we think that these ICU patients are representatives of the aSAH population where brain monitoring might have additional value. Furthermore, the recordings were limited to 90 min of data collection, and different observations might occur with longer EVD closure periods or at least differentiate between patients being EVD dependent. However, 30 min EVD closure seems reasonable for patient safety and reliable CA calculation in a well-controlled situation. It is important to mention that we used the most proximal exit of the EVD for our recordings in order to limit the dampening effect of the EVD tubing with CSF drainage. A study in 2007 where ICP measurement was performed from the distal part of drainage system (just before drip chamber) suggested that ICP measurements with open drain were unreliable and recommended independent parenchymal transducers for ICP monitoring after aSAH (Fig. 1c) [10].
Conclusion
Due to preserved slow fluctuations in the ICP signal, an open EVD system can be used to calculate dynamic autoregulation indices in aSAH patients requiring IC monitoring with the pressure measurement from the most proximal part of drain. If these results are confirmed in larger study, this technique can open the way for investigating the role of autoregulation disturbance in aSAH patients.
References
Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke. 1997;28(3):660–4.
Minhas PS, Menon DK, Smielewski P, et al. Positron emission tomographic cerebral perfusion disturbances and transcranial Doppler findings among patients with neurological deterioration after subarachnoid hemorrhage. Neurosurgery. 2003;52(5):1017–22.
Dhar R, Scalfani MT, Blackburn S, Zazulia AR, Videen T, Diringer M. Relationship between angiographic vasospasm and regional hypoperfusion in aneurysmal subarachnoid hemorrhage. Stroke. 2012;43(7):1788–94.
Macdonald RL, Pluta RM, Zhang JH. Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat Clin Pract Neurol. 2007;3(5):256–63.
Le RP, Menon DK, Citerio G, et al. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(9):1189–209.
Jaeger M, Schuhmann MU, Soehle M, Nagel C, Meixensberger J. Continuous monitoring of cerebrovascular autoregulation after subarachnoid hemorrhage by brain tissue oxygen pressure reactivity and its relation to delayed cerebral infarction. Stroke. 2007;38(3):981–6.
Lam JM, Smielewski P, Czosnyka M, Pickard JD, Kirkpatrick PJ. Predicting delayed ischemic deficits after aneurysmal subarachnoid hemorrhage using a transient hyperemic response test of cerebral autoregulation. Neurosurgery. 2000;47(4):819–25.
Jaeger M, Soehle M, Schuhmann MU, Meixensberger J. Clinical significance of impaired cerebrovascular autoregulation after severe aneurysmal subarachnoid hemorrhage. Stroke. 2012;43(8):2097–101.
Budohoski KP, Czosnyka M, Kirkpatrick PJ, Smielewski P, Steiner LA, Pickard JD. Clinical relevance of cerebral autoregulation following subarachnoid haemorrhage. Nat Rev Neurol. 2013;9(3):152–63.
Soehle M, Chatfield DA, Czosnyka M, Kirkpatrick PJ. Predictive value of initial clinical status, intracranial pressure and transcranial Doppler pulsatility after subarachnoid haemorrhage. Acta Neurochir (Wien). 2007;149(6):575–83.
Birch AA, Eynon CA, Schley D. Erroneous intracranial pressure measurements from simultaneous pressure monitoring and ventricular drainage catheters. Neurocrit Care. 2006;5(1):51–4.
Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 2013;35(2):93–112.
Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1997;41(1):11–7.
Aries MJ, Czosnyka M, Budohoski KP, et al. Continuous monitoring of cerebrovascular reactivity using pulse waveform of intracranial pressure. Neurocrit Care. 2012;17(1):67–76.
Howells T, Johnson U, McKelvey T, Enblad P. An optimal frequency range for assessing the pressure reactivity index in patients with traumatic brain injury. J Clin Monit Comput. 2014. doi:10.1007/s10877-014-9573-7.
Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data–theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol. 1995;64(2–3):237–78.
Holm S, Eide PK. The frequency domain versus time domain methods for processing of intracranial pressure (ICP) signals. Med Eng Phys. 2008;30(2):164–70.
Czosnyka M, Guazzo E, Whitehouse M, et al. Significance of intracranial pressure waveform analysis after head injury. Acta Neurochir (Wien). 1996;138(5):531–41.
Czosnyka M, Brady K, Reinhard M, Smielewski P, Steiner LA. Monitoring of cerebrovascular autoregulation: facts, myths, and missing links. Neurocrit Care. 2009;10(3):373–86.
Wilkinson HA, Yarzebski J, Wilkinson EC, Anderson FA Jr. Erroneous measurement of intracranial pressure caused by simultaneous ventricular drainage: a hydrodynamic model study. Neurosurgery. 1989;24(3):348–54.
Kim GS, Amato A, James ML, et al. Continuous and intermittent CSF diversion after subarachnoid hemorrhage: a pilot study. Neurocrit Care. 2011;14(1):68–72.
Aries MJ, Czosnyka M, Budohoski KP, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012;40:2456–63.
Conflict of interests
The software for brain monitoring ICM+ (www.neurosurg.cam.ac.uk/imcplus) is licensed by the University of Cambridge (Cambridge Enterprise). Peter Smielewski and Marek Czosnyka have a financial interest in a part of the licensing fee. Marcel JH Aries received a travel grant from freemason Lodge “L’ Union Provinciale” Groningen. Jan Willem Elting, Joost Regtien, Marc van Dijk, Sytse de Jong, Bart Depreitere declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aries, M.J.H., de Jong, S.F., van Dijk, J.M.C. et al. Observation of Autoregulation Indices During Ventricular CSF Drainage After Aneurysmal Subarachnoid Hemorrhage: A Pilot Study. Neurocrit Care 23, 347–354 (2015). https://doi.org/10.1007/s12028-015-0107-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-015-0107-z