Abstract
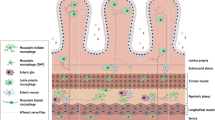
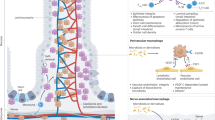
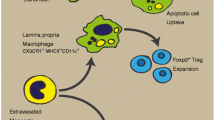
Muscularis macrophages, as the most abundant immune cells in the intestinal muscularis externa, exhibit tissue protective phenotype in the steady state. Owing to tremendous advances in technology, we now know the fact that muscularis macrophages are a heterogeneous population of cells which could be divided into different functional subsets depending on their anatomic niches. There is emerging evidence showing that these subsets, through molecular interactions with their neighbours, take part in a wide range of physiological and pathophysiological processes in the gut. In this review, we summarize recent progress (particularly over the past 4 years) on distribution, morphology, origin and functions of muscularis macrophages and, where possible, the characteristics of specific subsets in response to the microenvironment they occupy, with particular emphasis on their role in muscular inflammation. Furthermore, we also integrate their role in inflammation-related gastrointestinal disorders, such as post-operative ileus and diabetic gastroparesis, in order to propose future therapeutic strategies.




Similar content being viewed by others
Data availability
The authors confirm that the data mentioned in this study are available within the cited article.
References
Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15:123–47. https://doi.org/10.1146/annurev-pathmechdis-012418-012718.
Sly LM, McKay DM. Macrophage immunotherapy: overcoming impediments to realize promise. Trends Immunol. 2022;43(12):959–68. https://doi.org/10.1016/j.it.2022.10.002.
Bleriot C, Chakarov S, Ginhoux F. Determinants of Resident Tissue Macrophage Identity and Function. Immunity. 2020;52(6):957–70. https://doi.org/10.1016/j.immuni.2020.05.014.
Murray PJ. On macrophage diversity and inflammatory metabolic timers. Nat Rev Immunol. 2020;20(2):89–90. https://doi.org/10.1038/s41577-019-0260-2.
Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–66. https://doi.org/10.1146/annurev-physiol-022516-034339.
Yang Z, Lin S, Feng W, Liu Y, Song Z, Pan G, et al. A potential therapeutic target in traditional Chinese medicine for ulcerative colitis: macrophage polarization. Front Pharmacol. 2022;13:999179. https://doi.org/10.3389/fphar.2022.999179.
Zundler S, Gunther C, Kremer AE, Zaiss MM, Rothhammer V, Neurath MF. Gut immune cell trafficking: inter-organ communication and immune-mediated inflammation. Nat Rev Gastroenterol Hepatol. 2023;20(1):50–64. https://doi.org/10.1038/s41575-022-00663-1.
Viola MF, Boeckxstaens G. Intestinal resident macrophages: multitaskers of the gut. Neurogastroenterol Motil. 2020;32(8):e13843. https://doi.org/10.1111/nmo.13843.
Muller PA, Matheis F, Mucida D. Gut macrophages: key players in intestinal immunity and tissue physiology. Curr Opin Immunol. 2020;62:54–61. https://doi.org/10.1016/j.coi.2019.11.011.
Yip JLK, Balasuriya GK, Spencer SJ, Hill-Yardin EL. The role of intestinal macrophages in gastrointestinal homeostasis: heterogeneity and implications in disease. Cell Mol Gastroenterol Hepatol. 2021;12(5):1701–18. https://doi.org/10.1016/j.jcmgh.2021.08.021.
Wang S, Ye Q, Zeng X, Qiao S. Functions of macrophages in the maintenance of intestinal homeostasis. J Immunol Res. 2019;2019:1512969. https://doi.org/10.1155/2019/1512969.
Wehner S, Engel DR. Resident macrophages in the healthy and inflamed intestinal muscularis externa. Pflugers Arch. 2017;469(3-4):541–52. https://doi.org/10.1007/s00424-017-1948-4.
De Schepper S, Stakenborg N, Matteoli G, Verheijden S, Boeckxstaens GE. Muscularis macrophages: key players in intestinal homeostasis and disease. Cell Immunol. 2018;330:142–50. https://doi.org/10.1016/j.cellimm.2017.12.009.
Stubbington MJT, Rozenblatt-Rosen O, Regev A, Teichmann SA. Single-cell transcriptomics to explore the immune system in health and disease. Science. 2017;358(6359):58–63. https://doi.org/10.1126/science.aan6828.
Viola MF, Boeckxstaens G. Muscularis macrophages: trained guardians of enteric neurons. Cell Res. 2022;32(3):229–30. https://doi.org/10.1038/s41422-021-00602-w.
Lee SE, Rudd BD, Smith NL. Fate-mapping mice: new tools and technology for immune discovery. Trends Immunol. 2022;43(3):195–209. https://doi.org/10.1016/j.it.2022.01.004.
Anwar SM, Abd-Elhafeez HH, Abdel-Maksoud FM, Abdalla KEH. Morph-anatomic and histochemical study of ileum of goose (Alopochen egyptiacus) with special references to immune cells, mucous and serous goblet cells, telocytes, and dark and light smooth muscle fibers. Microsc Res Tech. 2021;84(6):1328–47. https://doi.org/10.1002/jemt.23692.
Arroyo Portilla C, Tomas J, Gorvel JP, Lelouard H. From species to regional and local specialization of intestinal macrophages. Front Cell Dev Biol. 2020;8:624213. https://doi.org/10.3389/fcell.2020.624213.
Graves CL, Chen A, Kwon V, Shiau CE. Zebrafish harbor diverse intestinal macrophage populations including a subset intimately associated with enteric neural processes. iScience. 2021;24(6):102496. https://doi.org/10.1016/j.isci.2021.102496.
Mikkelsen HB. Macrophages in the external muscle layers of mammalian intestines. Histol Histopathol. 1995;10(3):719–36.
Mikkelsen HB, Larsen JO, Froh P, Nguyen TH. Quantitative assessment of macrophages in the muscularis externa of mouse intestines. Anat Rec (Hoboken). 2011;294(9):1557–65. https://doi.org/10.1002/ar.21444.
Qian H, Wang Y, Chen X, Lin L, Zhang W, Wang Y, et al. “M1/M2” muscularis macrophages are associated with reduction of interstitial cells of cajal and glial cells in achalasia. Dig Dis Sci. 2022;68(4):1260–8. https://doi.org/10.1007/s10620-022-07734-y.
Kim SD, Kim IG, Tran HN, Cho H, Janarthanan G, Noh I, et al. Three-dimensional printed design of antibiotic-releasing esophageal patches for antimicrobial activity prevention. Tissue Eng Part A. 2021;27(23-24):1490–502. https://doi.org/10.1089/ten.TEA.2020.0268.
Rhee S, Yamamoto M, Kitamura K, Masaaki K, Katori Y, Murakami G, et al. Macrophage density in pharyngeal and laryngeal muscles greatly exceeds that in other striated muscles: an immunohistochemical study using elderly human cadavers. Anat Cell Biol. 2016;49(3):177–83. https://doi.org/10.5115/acb.2016.49.3.177.
Becker L, Nguyen L, Gill J, Kulkarni S, Pasricha PJ, Habtezion A. Age-dependent shift in macrophage polarisation causes inflammation-mediated degeneration of enteric nervous system. Gut. 2018;67(5):827–36. https://doi.org/10.1136/gutjnl-2016-312940.
Moura Silva H, Kitoko JZ, Queiroz CP, Kroehling L, Matheis F, Yang KL, et al. c-MAF-dependent perivascular macrophages regulate diet-induced metabolic syndrome. Sci Immunol. 2021;6(64):eabg7506. https://doi.org/10.1126/sciimmunol.abg7506.
Ji S, Traini C, Mischopoulou M, Gibbons SJ, Ligresti G, Faussone-Pellegrini MS, et al. Muscularis macrophages establish cell-to-cell contacts with telocytes/PDGFRalpha-positive cells and smooth muscle cells in the human and mouse gastrointestinal tract. Neurogastroenterol Motil. 2021;33(3):e13993. https://doi.org/10.1111/nmo.13993.
Sternini C. Structural and chemical organization of the myenteric plexus. Annu Rev Physiol. 1988;50:81–93. https://doi.org/10.1146/annurev.ph.50.030188.000501.
Mikkelsen HB, Rumessen JJ. Characterization of macrophage-like cells in the external layers of human small and large intestine. Cell Tissue Res. 1992;270(2):273–9. https://doi.org/10.1007/BF00328013.
Dora D, Ferenczi S, Stavely R, Toth VE, Varga ZV, Kovacs T, et al. Evidence of a myenteric plexus barrier and its macrophage-dependent degradation during murine colitis: implications in enteric neuroinflammation. Cell Mol Gastroenterol Hepatol. 2021;12(5):1617–41. https://doi.org/10.1016/j.jcmgh.2021.07.003.
Phillips RJ, Powley TL. Macrophages associated with the intrinsic and extrinsic autonomic innervation of the rat gastrointestinal tract. Auton Neurosci. 2012;169(1):12–27. https://doi.org/10.1016/j.autneu.2012.02.004.
Dora D, Arciero E, Hotta R, Barad C, Bhave S, Kovacs T, et al. Intraganglionic macrophages: a new population of cells in the enteric ganglia. J Anat. 2018;233(4):401–10. https://doi.org/10.1111/joa.12863.
Kulkarni S, Micci MA, Leser J, Shin C, Tang SC, Fu YY, et al. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc Natl Acad Sci U S A. 2017;114(18):E3709–E18. https://doi.org/10.1073/pnas.1619406114.
Avetisyan M, Rood JE, Huerta Lopez S, Sengupta R, Wright-Jin E, Dougherty JD, et al. Muscularis macrophage development in the absence of an enteric nervous system. Proc Natl Acad Sci U S A. 2018;115(18):4696–701. https://doi.org/10.1073/pnas.1802490115.
Lisowski ZM, Sauter KA, Waddell LA, Hume DA, Pirie RS, Hudson NPH. Immunohistochemical study of morphology and distribution of CD163(+ve) macrophages in the normal adult equine gastrointestinal tract. Vet Immunol Immunopathol. 2020;226:110073. https://doi.org/10.1016/j.vetimm.2020.110073.
Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell. 2016;164(3):378–91. https://doi.org/10.1016/j.cell.2015.12.023.
Enderes J, Mallesh S, Schneider R, Hupa KJ, Lysson M, Schneiker B, et al. A population of radio-resistant macrophages in the deep myenteric plexus contributes to postoperative ileus via toll-like receptor 3 signaling. Front Immunol. 2020;11:581111. https://doi.org/10.3389/fimmu.2020.581111.
Nemethova A, Michel K, Gomez-Pinilla PJ, Boeckxstaens GE, Schemann M. Nicotine attenuates activation of tissue resident macrophages in the mouse stomach through the beta2 nicotinic acetylcholine receptor. PloS one. 2013;8(11):e79264. https://doi.org/10.1371/journal.pone.0079264.
Mikkelsen HB, Garbarsch C, Tranum-Jensen J, Thuneberg L. Macrophages in the small intestinal muscularis externa of embryos, newborn and adult germ-free mice. J Mol Histol. 2004;35(4):377–87. https://doi.org/10.1023/b:hijo.0000039840.86420.b7.
Stremmel C, Schuchert R, Wagner F, Thaler R, Weinberger T, Pick R, et al. Yolk sac macrophage progenitors traffic to the embryo during defined stages of development. Nat Commun. 2018;9(1):75. https://doi.org/10.1038/s41467-017-02492-2.
Mass E. Delineating the origins, developmental programs and homeostatic functions of tissue-resident macrophages. Int Immunol. 2018;30(11):493–501. https://doi.org/10.1093/intimm/dxy044.
Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. https://doi.org/10.1016/j.immuni.2012.12.001.
De Schepper S, Verheijden S, Aguilera-Lizarraga J, Viola MF, Boesmans W, Stakenborg N, et al. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell. 2018;175(2):400–15 e13. https://doi.org/10.1016/j.cell.2018.07.048.
Domanska D, Majid U, Karlsen VT, Merok MA, Beitnes AR, Yaqub S, et al. Single-cell transcriptomic analysis of human colonic macrophages reveals niche-specific subsets. J Exp Med. 2022;219(3) https://doi.org/10.1084/jem.20211846.
Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6(3):498–510. https://doi.org/10.1038/mi.2012.89.
Bernardo D, Marin AC, Fernandez-Tome S, Montalban-Arques A, Carrasco A, Tristan E, et al. Human intestinal pro-inflammatory CD11c(high)CCR2(+)CX3CR1(+) macrophages, but not their tolerogenic CD11c(-)CCR2(-)CX3CR1(-) counterparts, are expanded in inflammatory bowel disease. Mucosal Immunol. 2018;11(4):1114–26. https://doi.org/10.1038/s41385-018-0030-7.
Batra A, Bui TM, Rehring JF, Yalom LK, Muller WA, Sullivan DP, et al. Experimental colitis enhances temporal variations in CX3CR1 cell colonization of the gut and brain following irradiation. Am J Pathol. 2022;192(2):295–307. https://doi.org/10.1016/j.ajpath.2021.10.013.
Chitu V, Stanley ER. Regulation of Embryonic and postnatal development by the CSF-1 Receptor. Curr Top Dev Biol. 2017;123:229–75. https://doi.org/10.1016/bs.ctdb.2016.10.004.
Cipriani G, Gibbons SJ, Miller KE, Yang DS, Terhaar ML, Eisenman ST, et al. Change in populations of macrophages promotes development of delayed gastric emptying in mice. Gastroenterology. 2018;154(8):2122–36 e12. https://doi.org/10.1053/j.gastro.2018.02.027.
Muller PA, Koscso B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158(2):300–13. https://doi.org/10.1016/j.cell.2014.04.050.
Drokhlyansky E, Smillie CS, Van Wittenberghe N, Ericsson M, Griffin GK, Eraslan G, et al. The human and mouse enteric nervous system at single-cell resolution. Cell. 2020;182(6):1606–22 e23. https://doi.org/10.1016/j.cell.2020.08.003.
Grubisic V, McClain JL, Fried DE, Grants I, Rajasekhar P, Csizmadia E, et al. Enteric glia modulate macrophage phenotype and visceral sensitivity following inflammation. Cell Rep. 2020;32(10):108100. https://doi.org/10.1016/j.celrep.2020.108100.
Cipriani G, Terhaar ML, Eisenman ST, Ji S, Linden DR, Wright AM, et al. Muscularis propria macrophages alter the proportion of nitrergic but not cholinergic gastric myenteric neurons. Cell Mol. Gastroenterol Hepatol. 2019;7(3):689–91 e4. https://doi.org/10.1016/j.jcmgh.2019.01.005.
Luo J, Qian A, Oetjen LK, Yu W, Yang P, Feng J, et al. TRPV4 channel signaling in macrophages promotes gastrointestinal motility via direct effects on smooth muscle cells. Immunity. 2018;49(1):107–19 e4. https://doi.org/10.1016/j.immuni.2018.04.021.
Mischopoulou M, D'Ambrosio M, Bigagli E, Luceri C, Farrugia G, Cipriani G. Role of macrophages and mast cells as key players in the maintenance of gastrointestinal smooth muscle homeostasis and disease. Cell Mol Gastroenterol Hepatol. 2022;13(6):1849–62. https://doi.org/10.1016/j.jcmgh.2022.02.017.
Wang H, Foong JPP, Harris NL, Bornstein JC. Correction to: Enteric neuroimmune interactions coordinate intestinal responses in health and disease. Mucosal Immunol. 2022;15(1):188. https://doi.org/10.1038/s41385-021-00459-7.
Phillips RJ, Billingsley CN, Powley TL. Macrophages are unsuccessful in clearing aggregated alpha-synuclein from the gastrointestinal tract of healthy aged Fischer 344 rats. Anat Rec (Hoboken). 2013;296(4):654–69. https://doi.org/10.1002/ar.22675.
Phillips RJ, Walter GC, Ringer BE, Higgs KM, Powley TL. Alpha-synuclein immunopositive aggregates in the myenteric plexus of the aging Fischer 344 rat. Exp Neurol. 2009;220(1):109–19. https://doi.org/10.1016/j.expneurol.2009.07.025.
Becker L, Spear ET, Sinha SR, Haileselassie Y, Habtezion A. Age-related changes in gut microbiota alter phenotype of muscularis macrophages and disrupt gastrointestinal motility. Cell Mol. Gastroenterol Hepatol. 2019;7(1):243–5 e2. https://doi.org/10.1016/j.jcmgh.2018.09.001.
Freire BM, de Melo FM, Basso AS. Adrenergic signaling regulation of macrophage function: do we understand it yet? Immunother Adv. 2022;2(1):ltac010. https://doi.org/10.1093/immadv/ltac010.
Jacobson A, Yang D, Vella M, Chiu IM. The intestinal neuro-immune axis: crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 2021;14(3):555–65. https://doi.org/10.1038/s41385-020-00368-1.
Zila I, Mokra D, Kopincova J, Kolomaznik M, Javorka M, Calkovska A. Vagal-immune interactions involved in cholinergic anti-inflammatory pathway. Physiol Res. 2017;66(Suppl 2):S139–S45. https://doi.org/10.33549/physiolres.933671.
Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005;19(6):493–9. https://doi.org/10.1016/j.bbi.2005.03.015.
Matteoli G, Gomez-Pinilla PJ, Nemethova A, Di Giovangiulio M, Cailotto C, van Bree SH, et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. 2014;63(6):938–48. https://doi.org/10.1136/gutjnl-2013-304676.
Matteoli G, Boeckxstaens GE. The vagal innervation of the gut and immune homeostasis. Gut. 2013;62(8):1214–22. https://doi.org/10.1136/gutjnl-2012-302550.
Cailotto C, Gomez-Pinilla PJ, Costes LM, van der Vliet J, Di Giovangiulio M, Nemethova A, et al. Neuro-anatomical evidence indicating indirect modulation of macrophages by vagal efferents in the intestine but not in the spleen. PloS one. 2014;9(1):e87785. https://doi.org/10.1371/journal.pone.0087785.
Matheis F, Muller PA, Graves CL, Gabanyi I, Kerner ZJ, Costa-Borges D, et al. Adrenergic signaling in muscularis macrophages limits infection-induced neuronal loss. Cell. 2020;180(1):64–78 e16. https://doi.org/10.1016/j.cell.2019.12.002.
Netea MG, Dominguez-Andres J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20(6):375–88. https://doi.org/10.1038/s41577-020-0285-6.
Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, et al. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098. https://doi.org/10.1126/science.aaf1098.
Osborne LC. Protecting your gut feelings: how intestinal infections keep things moving. Neuron. 2021;109(22):3545–7. https://doi.org/10.1016/j.neuron.2021.10.037.
Tsuchida Y, Hatao F, Fujisawa M, Murata T, Kaminishi M, Seto Y, et al. Neuronal stimulation with 5-hydroxytryptamine 4 receptor induces anti-inflammatory actions via alpha7nACh receptors on muscularis macrophages associated with postoperative ileus. Gut. 2011;60(5):638–47. https://doi.org/10.1136/gut.2010.227546.
Farro G, Stakenborg M, Gomez-Pinilla PJ, Labeeuw E, Goverse G, Di Giovangiulio M, et al. CCR2-dependent monocyte-derived macrophages resolve inflammation and restore gut motility in postoperative ileus. Gut. 2017;66(12):2098–109. https://doi.org/10.1136/gutjnl-2016-313144.
Yuan PQ, Tache Y. Abdominal surgery induced gastric ileus and activation of M1-like macrophages in the gastric myenteric plexus: prevention by central vagal activation in rats. Am J Physiol Gastrointest Liver Physiol. 2017;313(4):G320–G9. https://doi.org/10.1152/ajpgi.00121.2017.
Grover M, Bernard CE, Pasricha PJ, Parkman HP, Gibbons SJ, Tonascia J, et al. Diabetic and idiopathic gastroparesis is associated with loss of CD206-positive macrophages in the gastric antrum. Neurogastroenterol Motil. 2017;29(6) https://doi.org/10.1111/nmo.13018.
Bernard CE, Gibbons SJ, Mann IS, Froschauer L, Parkman HP, Harbison S, et al. Association of low numbers of CD206-positive cells with loss of ICC in the gastric body of patients with diabetic gastroparesis. Neurogastroenterol Motil. 2014;26(9):1275–84. https://doi.org/10.1111/nmo.12389.
Grover M, Gibbons SJ, Nair AA, Bernard CE, Zubair AS, Eisenman ST, et al. Transcriptomic signatures reveal immune dysregulation in human diabetic and idiopathic gastroparesis. BMC Med Genomics. 2018;11(1):62. https://doi.org/10.1186/s12920-018-0379-1.
Grover M, Dasari S, Bernard CE, Chikkamenahalli LL, Yates KP, Pasricha PJ, et al. Proteomics in gastroparesis: unique and overlapping protein signatures in diabetic and idiopathic gastroparesis. Am J Physiol Gastrointest Liver Physiol. 2019;317(5):G716–G26. https://doi.org/10.1152/ajpgi.00115.2019.
Tian L, Song S, Zhu B, Liu S. Electroacupuncture at ST-36 Protects Interstitial Cells of Cajal via Sustaining Heme Oxygenase-1 Positive M2 Macrophages in the Stomach of Diabetic Mice. Oxid Med Cell Longev. 2018;2018:3987134. https://doi.org/10.1155/2018/3987134.
Wang H, Zhao K, Ba Y, Gao T, Shi N, Niu Q, et al. Gastric electrical pacing reduces apoptosis of interstitial cells of cajal via antioxidative stress effect attributing to phenotypic polarization of M2 macrophages in diabetic rats. Oxid Med Cell Longev. 2021;2021:1298657. https://doi.org/10.1155/2021/1298657.
Sui C, Tao L, Bai C, Shao L, Miao J, Chen K, et al. Molecular and cellular mechanisms underlying postoperative paralytic ileus by various immune cell types. Front Pharmacol. 2022;13:929901. https://doi.org/10.3389/fphar.2022.929901.
Vather R, O'Grady G, Bissett IP, Dinning PG. Postoperative ileus: mechanisms and future directions for research. Clin Exp Pharmacol Physiol. 2014;41(5):358–70. https://doi.org/10.1111/1440-1681.12220.
Wehner S, Behrendt FF, Lyutenski BN, Lysson M, Bauer AJ, Hirner A, et al. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut. 2007;56(2):176–85. https://doi.org/10.1136/gut.2005.089615.
Docsa T, Bhattarai D, Sipos A, Wade CE, Cox CS Jr, Uray K. CXCL1 is upregulated during the development of ileus resulting in decreased intestinal contractile activity. Neurogastroenterol Motil. 2020;32(3):e13757. https://doi.org/10.1111/nmo.13757.
Sun Y, Shi H, Hong Z, Chi P. Inhibition of JAK1 mitigates postoperative ileus in mice. Surgery. 2019;166(6):1048–54. https://doi.org/10.1016/j.surg.2019.07.016.
Stakenborg M, Abdurahiman S, De Simone V, Goverse G, Stakenborg N, van Baarle L, et al. Enteric glial cells favor accumulation of anti-inflammatory macrophages during the resolution of muscularis inflammation. Mucosal Immunol. 2022;15(6):1296–308. https://doi.org/10.1038/s41385-022-00563-2.
Hupa KJ, Stein K, Schneider R, Lysson M, Schneiker B, Hornung V, et al. AIM2 inflammasome-derived IL-1beta induces postoperative ileus in mice. Sci Rep. 2019;9(1):10602. https://doi.org/10.1038/s41598-019-46968-1.
Hussain Z, Park H. Inflammation and impaired gut physiology in post-operative ileus: mechanisms and the treatment options. J Neurogastroenterol Motil. 2022;28(4):517–30. https://doi.org/10.5056/jnm22100.
Matsumoto K, Kawanaka H, Hori M, Kusamori K, Utsumi D, Tsukahara T, et al. Role of transient receptor potential melastatin 2 in surgical inflammation and dysmotility in a mouse model of postoperative ileus. Am J Physiol Gastrointest Liver Physiol. 2018;315(1):G104–G16. https://doi.org/10.1152/ajpgi.00305.2017.
Pohl JM, Gutweiler S, Thiebes S, Volke JK, Klein-Hitpass L, Zwanziger D, et al. Irf4-dependent CD103(+)CD11b(+) dendritic cells and the intestinal microbiome regulate monocyte and macrophage activation and intestinal peristalsis in postoperative ileus. Gut. 2017;66(12):2110–20. https://doi.org/10.1136/gutjnl-2017-313856.
Schneider R, Leven P, Mallesh S, Bresser M, Schneider L, Mazzotta E, et al. IL-1-dependent enteric gliosis guides intestinal inflammation and dysmotility and modulates macrophage function. Commun Biol. 2022;5(1):811. https://doi.org/10.1038/s42003-022-03772-4.
Kalff JC, Buchholz BM, Eskandari MK, Hierholzer C, Schraut WH, Simmons RL, et al. Biphasic response to gut manipulation and temporal correlation of cellular infiltrates and muscle dysfunction in rat. Surgery. 1999;126(3):498–509.
Kalff JC, Carlos TM, Schraut WH, Billiar TR, Simmons RL, Bauer AJ. Surgically induced leukocytic infiltrates within the rat intestinal muscularis mediate postoperative ileus. Gastroenterology. 1999;117(2):378–87. https://doi.org/10.1053/gast.1999.0029900378.
The FO, de Jonge WJ, Bennink RJ, van den Wijngaard RM, Boeckxstaens GE. The ICAM-1 antisense oligonucleotide ISIS-3082 prevents the development of postoperative ileus in mice. Br J Pharmacol. 2005;146(2):252–8. https://doi.org/10.1038/sj.bjp.0706303.
Stoffels B, Schmidt J, Nakao A, Nazir A, Chanthaphavong RS, Bauer AJ. Role of interleukin 10 in murine postoperative ileus. Gut. 2009;58(5):648–60. https://doi.org/10.1136/gut.2008.153288.
Stein K, Lysson M, Schumak B, Vilz T, Specht S, Heesemann J, et al. Leukocyte-derived interleukin-10 aggravates postoperative ileus. Front Immunol. 2018;9:2599. https://doi.org/10.3389/fimmu.2018.02599.
Kimura H, Imura YK, Tomiyasu H, Mihara T, Kaji N, Ohno K, et al. Neural anti-inflammatory action mediated by two types of acetylcholine receptors in the small intestine. Sci Rep. 2019;9(1):5887. https://doi.org/10.1038/s41598-019-41698-w.
Stakenborg N, Labeeuw E, Gomez-Pinilla PJ, De Schepper S, Aerts R, Goverse G, et al. Preoperative administration of the 5-HT4 receptor agonist prucalopride reduces intestinal inflammation and shortens postoperative ileus via cholinergic enteric neurons. Gut. 2019;68(8):1406–16. https://doi.org/10.1136/gutjnl-2018-317263.
Milne T, Liu C, O'Grady G, Woodfield J, Bissett I. Effect of prucalopride to improve time to gut function recovery following elective colorectal surgery: randomized clinical trial. Br J Surg. 2022;109(8):704–10. https://doi.org/10.1093/bjs/znac121.
Zhang L, Wu Z, Zhou J, Lu S, Wang C, Xia Y, et al. Electroacupuncture ameliorates acute pancreatitis: a role for the vagus nerve-mediated cholinergic anti-inflammatory pathway. Front Mol Biosci. 2021;8:647647. https://doi.org/10.3389/fmolb.2021.647647.
Wang M, Pan W, Xu Y, Zhang J, Wan J, Jiang H. Microglia-mediated neuroinflammation: a potential target for the treatment of cardiovascular diseases. J Inflamm Res. 2022;15:3083–94. https://doi.org/10.2147/JIR.S350109.
Song S, An J, Liu S. Electroacupuncture accelerates the delayed intestinal transit in POI by suppressing M1 like muscularis macrophages and IL6 secretion. Neurogastroenterol Motil. 2021;33(6):e14066. https://doi.org/10.1111/nmo.14066.
Yang NN, Yang JW, Ye Y, Huang J, Wang L, Wang Y, et al. Electroacupuncture ameliorates intestinal inflammation by activating alpha7nAChR-mediated JAK2/STAT3 signaling pathway in postoperative ileus. Theranostics. 2021;11(9):4078–89. https://doi.org/10.7150/thno.52574.
Wang Y, Yang JW, Yan SY, Lu Y, Han JG, Pei W, et al. Electroacupuncture vs sham electroacupuncture in the treatment of postoperative ileus after laparoscopic surgery for colorectal cancer: a multicenter, randomized clinical trial. JAMA Surg. 2022;158(1):20–7. https://doi.org/10.1001/jamasurg.2022.5674.
Glowka TR, Steinebach A, Stein K, Schwandt T, Lysson M, Holzmann B, et al. The novel CGRP receptor antagonist BIBN4096BS alleviates a postoperative intestinal inflammation and prevents postoperative ileus. Neurogastroenterol Motil. 2015;27(7):1038–49. https://doi.org/10.1111/nmo.12584.
Mallesh S, Schneider R, Schneiker B, Lysson M, Efferz P, Lin E, et al. Sympathetic denervation alters the inflammatory response of resident muscularis macrophages upon surgical trauma and ameliorates postoperative ileus in mice. Int J Mol Sci. 2021;22(13) https://doi.org/10.3390/ijms22136872.
Camilleri M, Sanders KM. Gastroparesis. Gastroenterology. 2022;162(1):68–87 e1. https://doi.org/10.1053/j.gastro.2021.10.028.
Bharucha AE, Kudva YC, Prichard DO. Diabetic gastroparesis. Endocr Rev. 2019;40(5):1318–52. https://doi.org/10.1210/er.2018-00161.
Grover M, Farrugia G, Stanghellini V. Gastroparesis: a turning point in understanding and treatment. Gut. 2019;68(12):2238–50. https://doi.org/10.1136/gutjnl-2019-318712.
Pasricha PJ, Grover M, Yates KP, Abell TL, Koch KL, McCallum RW, et al. Progress in gastroparesis - a narrative review of the work of the Gastroparesis Clinical Research Consortium. Clin Gastroenterol Hepatol. 2022;20(12):2684–95 e3. https://doi.org/10.1016/j.cgh.2022.05.022.
Choi KM, Gibbons SJ, Nguyen TV, Stoltz GJ, Lurken MS, Ordog T, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135(6):2055–64. https://doi.org/10.1053/j.gastro.2008.09.003.
Cipriani G, Gibbons SJ, Verhulst PJ, Choi KM, Eisenman ST, Hein SS, et al. Diabetic Csf1(op/op) mice lacking macrophages are protected against the development of delayed gastric emptying. Cell Mol Gastroenterol Hepatol. 2016;2(1):40–7. https://doi.org/10.1016/j.jcmgh.2015.09.001.
Choi KM, Kashyap PC, Dutta N, Stoltz GJ, Ordog T, Shea Donohue T, et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138(7):2399–409. https://doi.org/10.1053/j.gastro.2010.02.014.
Gibbons SJ, Grover M, Choi KM, Wadhwa A, Zubair A, Wilson LA, et al. Repeat polymorphisms in the Homo sapiens heme oxygenase-1 gene in diabetic and idiopathic gastroparesis. PloS one. 2017;12(11):e0187772. https://doi.org/10.1371/journal.pone.0187772.
Bharucha AE, Daley SL, Low PA, Gibbons SJ, Choi KM, Camilleri M, et al. Effects of hemin on heme oxygenase-1, gastric emptying, and symptoms in diabetic gastroparesis. Neurogastroenterol Motil. 2016;28(11):1731–40. https://doi.org/10.1111/nmo.12874.
Chen Y, Xu JJ, Liu S, Hou XH. Electroacupuncture at ST36 ameliorates gastric emptying and rescues networks of interstitial cells of Cajal in the stomach of diabetic rats. PloS one. 2013;8(12):e83904. https://doi.org/10.1371/journal.pone.0083904.
Chen Y, Xu J, Liu S, Hou X. Electroacupuncture at ST36 increases contraction of the gastric antrum and improves the SCF/c-kit pathway in diabetic rats. Am J Chin Med. 2013;41(6):1233–49. https://doi.org/10.1142/S0192415X13500833.
Funding
This work was supported by grants from the Henan Province Foundation for University Key Youth Scholars (NO. 2017GGJS107), the Key Scientific and Technological Project of Henan Province (NO. 202102310348), the Teaching and Scientific Research Cultivation Project of Basic Medical College of Xinxiang Medical University (NO. JCYXYKY202018), and the Doctor Scientific Research Foundation of Xinxiang Medical University (NO. 300505276).
Author information
Authors and Affiliations
Contributions
LZ drafted the manuscript. HL, YY, YZ, YH, and GL contributed to the literature review, figure, and table design. ZW made the final editing and revision. All authors critically revised, and provided the final approval for this manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Informed consent
Not applicable.
Conflict of interest
The authors declare that the review was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, L., Lian, H., Yin, Y. et al. New insights into muscularis macrophages in the gut: from their origin to therapeutic targeting. Immunol Res 71, 785–799 (2023). https://doi.org/10.1007/s12026-023-09397-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-023-09397-x