Abstract
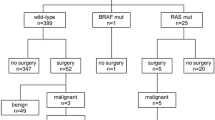
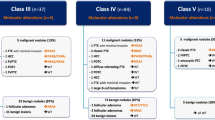
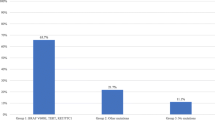
In cytologically indeterminate thyroid nodules undergoing molecular testing, estimated risk of malignancy is variable. Identification of a non-cancer-specific mutation (RAS-like) confirms a neoplastic process but does not differentiate between benign, malignant, and low-risk neoplasms. This study aims to retrospectively evaluate institutional experience of Interpace (ThyGeNEXT® and ThyraMIR®; Pittsburgh, PA) testing and to determine the rate of malignancy in resected nodules, stratified by mutational analysis and microRNA profile. Of 1917 fine need aspirations, 140 (7.3%) underwent Interpace testing: 47 (33.6%) were molecular-not-benign (harbored mutation, fusion, and/or positive miRNA) and 93 (66.4%) were molecular-benign (no mutations or fusions and negative microRNA). Surgery was spared in 79.6% of molecular-benign and 61.4% of all tested patients. Fifty-four (38.6%) underwent resection. Seventeen (89.5%) of the resected molecular-benign were benign and 2 were malignant. Thirteen (37.1%) of the resected molecular-not-benign were benign, 7 (20%) were noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), and 15 (42.9%) were malignant (p < 0.05, negative predictive value (NPV) 89.4–95.6%, positive predictive value (PPV) 22.3–42.8%). Most molecular-not-benign (72.3%) had RAS-like mutation. Twenty-three were resected: 3 were malignant and 7 were NIFTP. Nodules with non-RAS-like mutations (BRAF V600E-like, others) were more likely to be malignant than RAS-like (H/N/KRAS, BRAF K601E) (p < 0.05, NPV 86.9–96.5%, PPV 100%). Most nodules had RAS-like mutations and most were benign or low-risk neoplasms (NIFTP). This study supports the role of histologic examination in the distinction of malignancy in RAS-like thyroid neoplasms and underscores the role of molecular testing in risk stratification, patient counseling, and operative management.



Similar content being viewed by others
Availability of Data and Materials
Data is provided in manuscript; additional details can be requested through corresponding author.
References
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L (2016) 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133. https://doi.org/10.1089/thy.2015.0020
Cibas ES, Ali SZ (2017) The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 27:1341–1346. https://doi.org/10.1089/thy.2017.0500
Bessey LJ, Lai NBK, Coorough NE, Chen H, Sippel RS (2013) The incidence of thyroid cancer by fine needle aspiration varies by age and gender. Journal of Surgical Research 184:761–765. https://doi.org/10.1016/j.jss.2013.03.086
Nishino M, Bellevicine C, Baloch Z (2021) Molecular Tests for Risk-Stratifying Cytologically Indeterminate Thyroid Nodules: An Overview of Commercially Available Testing Platforms in the United States. Journal of Molecular Pathology 2:135–146. https://doi.org/10.3390/jmp2020014
Nishino M, Krane JF (2020) Role of Ancillary Techniques in Thyroid Cytology Specimens. Acta Cytologica 64:40–51
Giordano TJ (2018) Genomic Hallmarks of Thyroid Neoplasia. Annu Rev Pathol Mech Dis 13:141–62. https://doi.org/10.1146/annurev-pathol-121808
Walts AE, Pao A, Sacks W, Bose S (2014) BRAF genetic heterogeneity in papillary thyroid carcinoma and its metastasis. Human Pathology 45:935–941. https://doi.org/10.1016/j.humpath.2013.12.005
Labourier E, Shifrin A, Busseniers AE, Lupo MA, Manganelli ML, Andruss B, Wylie D, Beaudenon-Huibregtse S (2015) Molecular testing for miRNA, mRNA, and DNA on fine-needle aspiration improves the preoperative diagnosis of thyroid nodules with indeterminate cytology. Journal of Clinical Endocrinology and Metabolism 100:. https://doi.org/10.1210/jc.2015-1158
Lupo MA, Walts AE, Sistrunk JW, Giordano TJ, Sadow PM, Massoll N, Campbell R, Jackson SA, Toney N, Narick CM, Kumar G, Mireskandari A, Finkelstein SD, Bose S (2020) Multiplatform molecular test performance in indeterminate thyroid nodules. Diagnostic Cytopathology 48:1254–1264. https://doi.org/10.1002/dc.24564
Agrawal N, Akbani R, Aksoy BA, Ally A, Arachchi H, Asa SL, Auman JT, Balasundaram M, Balu S, Baylin SB, Behera M, Bernard B, Beroukhim R, Bishop JA, Black AD, Bodenheimer T, Boice L, Bootwalla MS, Bowen J, Bowlby R, Bristow CA, Brookens R, Brooks D, Bryant R, Buda E, Butterfield YSN, Carling T, Carlsen R, Carter SL, Carty SE, Chan TA, Chen AY, Cherniack AD, Cheung D, Chin L, Cho J, Chu A, Chuah E, Cibulskis K, Ciriello G, Clarke A, Clayman GL, Cope L, Copland JA, Covington K, Danilova L, Davidsen T, Demchok JA, DiCara D, Dhalla N, Dhir R, Dookran SS, Dresdner G, Eldridge J, Eley G, El-Naggar AK, Eng S, Fagin JA, Fennell T, Ferris RL, Fisher S, Frazer S, Frick J, Gabriel SB, Ganly I, Gao J, Garraway LA, Gastier-Foster JM, Getz G, Gehlenborg N, Ghossein R, Gibbs RA, Giordano TJ, Gomez-Hernandez K, Grimsby J, Gross B, Guin R, Hadjipanayis A, Harper HA, Hayes DN, Heiman DI, Herman JG, Hoadley KA, Hofree M, Holt RA, Hoyle AP, Huang FW, Huang M, Hutter CM, Ideker T, Iype L, Jacobsen A, Jefferys SR, Jones CD, Jones SJM, Kasaian K, Kebebew E, Khuri FR, Kim J, Kramer R, Kreisberg R, Kucherlapati R, Kwiatkowski DJ, Ladanyi M, Lai PH, Laird PW, Lander E, Lawrence MS, Lee D, Lee E, Lee S, Lee W, Leraas KM, Lichtenberg TM, Lichtenstein L, Lin P, Ling S, Liu J, Liu W, Liu Y, LiVolsi VA, Lu Y, Ma Y, Mahadeshwar HS, Marra MA, Mayo M, McFadden DG, Meng S, Meyerson M, Mieczkowski PA, Miller M, Mills G, Moore RA, Mose LE, Mungall AJ, Murray BA, Nikiforov YE, Noble MS, Ojesina AI, Owonikoko TK, Ozenberger BA, Pantazi A, Parfenov M, Park PJ, Parker JS, Paull EO, Pedamallu CS, Perou CM, Prins JF, Protopopov A, Ramalingam SS, Ramirez NC, Ramirez R, Raphael BJ, Rathmell WK, Ren X, Reynolds SM, Rheinbay E, Ringel MD, Rivera M, Roach J, Robertson AG, Rosenberg MW, Rosenthal M, Sadeghi S, Saksena G, Sander C, Santoso N, Schein JE, Schultz N, Schumacher SE, Seethala RR, Seidman J, Senbabaoglu Y, Seth S, Sharpe S, Shaw KRM, Shen JP, Shen R, Sherman S, Sheth M, Shi Y, Shmulevich I, Sica GL, Simons J v., Sinha R, Sipahimalani P, Smallridge RC, Sofia HJ, Soloway MG, Song X, Sougnez C, Stewart C, Stojanov P, Stuart JM, Sumer SO, Sun Y, Tabak B, Tam A, Tan D, Tang J, Tarnuzzer R, Taylor BS, Thiessen N, Thorne L, Thorsson V, Tuttle RM, Umbricht CB, van den Berg DJ, Vandin F, Veluvolu U, Verhaak RGW, Vinco M, Voet D, Walter V, Wang Z, Waring S, Weinberger PM, Weinhold N, Weinstein JN, Weisenberger DJ, Wheeler D, Wilkerson MD, Wilson J, Williams M, Winer DA, Wise L, Wu J, Xi L, Xu AW, Yang L, Yang L, Zack TI, Zeiger MA, Zeng D, Zenklusen JC, Zhao N, Zhang H, Zhang J, Zhang J, Zhang W, Zmuda E, Zou L (2014) Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 159:676–690. https://doi.org/10.1016/j.cell.2014.09.050
Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, LiVolsi VA, Papotti MG, Sobrinho-Simões M, Tallini G, Mete O (2022) Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocrine Pathology 33:27–63
Frates MC, Benson CB, Doubilet PM, Kunreuther E, Contreras M, Cibas ES, Orcutt J, Moore FD, Larsen PR, Marqusee E, Alexander EK (2006) Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. Journal of Clinical Endocrinology and Metabolism 91:3411–3417. https://doi.org/10.1210/jc.2006-0690
Johnson DN, Cavallo AB, Uraizee I, Tanager K, Lastra RR, Antic T, Cipriani NA (2019) A Proposal for Separation of Nuclear Atypia and Architectural Atypia in Bethesda Category III (AUS/FLUS) Based on Differing Rates of Thyroid Malignancy. American Journal of Clinical Pathology 151:. https://doi.org/10.1093/ajcp/aqy109
Hu MI, Waguespack SG, Dosiou C, Ladenson PW, Livhits MJ, Wirth LJ, Sadow PM, Krane JF, Stack BC, Zafereo ME, Ali SZ, Weitzman SP, Hao Y, Babiarz JE, Kennedy GC, Kloos RT (2021) Afirma Genomic Sequencing Classifier and Xpression Atlas Molecular Findings in Consecutive Bethesda III-VI Thyroid Nodules. Journal of Clinical Endocrinology and Metabolism 106:2198–2207. https://doi.org/10.1210/clinem/dgab304
Banizs AB, Silverman JF (2019) The utility of combined mutation analysis and microRNA classification in reclassifying cancer risk of cytologically indeterminate thyroid nodules. Diagnostic Cytopathology 47:268–274. https://doi.org/10.1002/dc.24087
Kakudo K (2022) Different Threshold of Malignancy for RAS-like Thyroid Tumors Causes Significant Differences in Thyroid Nodule Practice. Cancers (Basel) 14
Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LDR, Barletta JA, Wenig BM, Ghuzlan A al, Kakudo K, Giordano TJ, Alves VA, Khanafshar E, Asa SL, El-Naggar AK, Gooding WE, Hodak SP, Lloyd R v., Maytal G, Mete O, Nikiforova MN, Nosé V, Papotti M, Poller DN, Sadow PM, Tischler AS, Michael TR, Wall KB, LiVolsi VA, Randolph GW, Ghossein RA (2016) Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncology 2:1023–1029. https://doi.org/10.1001/jamaoncol.2016.0386
Nikiforov YE, Baloch ZW, Hodak SP, Giordano TJ, Lloyd R v., Seethala RR, Wenig BM (2018) Change in diagnostic criteria for noninvasive follicular thyroid neoplasm with papillarylike nuclear features. JAMA Oncology 4:1125–1126
Patel SG, Carty SE, McCoy KL, Ohori NP, LeBeau SO, Seethala RR, Nikiforova MN, Nikiforov YE, Yip L (2017) Preoperative detection of RAS mutation may guide extent of thyroidectomy. In: Surgery (United States)
An JH, Song KH, Kim SK, Park KS, Yoo YB, Yang JH, Hwang TS, Kim DL (2015) RAS mutations in indeterminate thyroid nodules are predictive of the follicular variant of papillary thyroid carcinoma. Clinical Endocrinology 82:760–766. https://doi.org/10.1111/cen.12579
Gupta N, Dasyam AK, Carty SE, Nikiforova MN, Ohori NP, Armstrong M, Yip L, LeBeau SO, McCoy KL, Coyne C, Stang MT, Johnson J, Ferris RL, Seethala R, Nikiforov YE, Hodak SP (2013) RAS mutations in thyroid FNA specimens are highly predictive of predominantly low-risk follicular-pattern cancers. Journal of Clinical Endocrinology and Metabolism 98:. https://doi.org/10.1210/jc.2012-3396
Hwang TS, Kim WY, Han HS, Lim SD, Kim WS, Yoo YB, Park KS, Oh SY, Kim SK, Yang JH (2015) Preoperative RAS mutational analysis is of great value in predicting follicular variant of papillary thyroid carcinoma. BioMed Research International 2015:. https://doi.org/10.1155/2015/697068
Medici M, Kwong N, Angell TE, Marqusee E, Kim MI, Frates MC, Benson CB, Cibas ES, Barletta JA, Krane JF, Ruan DT, Cho NL, Gawande AA, Moore FD, Alexander EK (2015) The variable phenotype and low-risk nature of RAS-positive thyroid nodules. BMC Medicine 13:. https://doi.org/10.1186/s12916-015-0419-z
Radkay LA, Chiosea SI, Seethala RR, Hodak SP, Lebeau SO, Yip L, McCoy KL, Carty SE, Schoedel KE, Nikiforova MN, Nikiforov YE, Ohori NP (2014) Thyroid nodules with KRAS mutations are different from nodules with NRAS and HRAS mutations with regard to cytopathologic and histopathologic outcome characteristics. Cancer Cytopathology 122:873–882. https://doi.org/10.1002/cncy.21474
Ravella L, Lopez J, Descotes F, Giai J, Lapras V, Denier ML, Borson-Chazot F, Lifante JC, Decaussin-Petrucci M (2020) Preoperative Role of RAS or BRAF K601E in the Guidance of Surgery for Indeterminate Thyroid Nodules. World Journal of Surgery 44:2264–2271. https://doi.org/10.1007/s00268-020-05487-1
Stence AA, Gailey MP, Robinson RA, Jensen CS, Ma D (2015) Simultaneously Detection of 50 Mutations at 20 Sites in the BRAF and RAS Genes by Multiplexed Single-Nucleotide Primer Extension Assay Using Fine-Needle Aspirates of Thyroid Nodules
Nikiforov YE, Ohori NP, Hodak SP, Carty SE, LeBeau SO, Ferris RL, Yip L, Seethala RR, Tublin ME, Stang MT, Coyne C, Johnson JT, Stewart AF, Nikiforova MN (2011) Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: A prospective analysis of 1056 FNA samples. Journal of Clinical Endocrinology and Metabolism 96:3390–3397. https://doi.org/10.1210/jc.2011-1469
Chin PD, Zhu CY, Sajed DP, Fishbein GA, Yeh MW, Leung AM, Livhits MJ (2022) Correlation of ThyroSeq Results with Surgical Histopathology in Cytologically Indeterminate Thyroid Nodules. https://doi.org/10.1007/s12022-020-09641-2/Published
Labourier E (2016) Utility and cost-effectiveness of molecular testing in thyroid nodules with indeterminate cytology. Clinical Endocrinology 85:624–631. https://doi.org/10.1111/cen.13096
Shapiro S, Pharaon M, Kellermeyer B (2017) Cost-effectiveness of Gene Expression Classifier Testing of Indeterminate Thyroid Nodules Utilizing a Real Cohort Comparator. Otolaryngology - Head and Neck Surgery (United States) 157:596–601. https://doi.org/10.1177/0194599817725709
Nicholson KJ, Roberts MS, McCoy KL, Carty SE, Yip L (2019) Molecular Testing Versus Diagnostic Lobectomy in Bethesda III/IV Thyroid Nodules: A Cost-Effectiveness Analysis. Thyroid 29:1237–1243. https://doi.org/10.1089/thy.2018.0779
Ronen O, Oichman M (2021) National differences in cost analysis of Afirma Genomic sequencing classifier. Clinical Endocrinology 94:717–724. https://doi.org/10.1111/cen.14400
Ontario Health (Quality) (2022) Molecular Testing for Thyroid Nodules of Indeterminate Cytology: A Health Technology Assessment. Ontario Health Technology Assessment Series 22:1–111
Satoh S, Yamashita H, Kakudo K (2017) Thyroid cytology: The Japanese system and experience at Yamashita Thyroid Hospital. Journal of Pathology and Translational Medicine 51:548–554. https://doi.org/10.4132/jptm.2017.09.29
Hirokawa M, Suzuki A, Kawakami M, Kudo T, Miyauchi A (2022) Criteria for follow-up of thyroid nodules diagnosed as follicular neoplasm without molecular testing – The experience of a high-volume thyroid centre in Japan. Diagnostic Cytopathology 50:223–229. https://doi.org/10.1002/dc.24937
Clinkscales W, Ong A, Nguyen S, Harruff EE, Gillespie MB (2017) Diagnostic Value of RAS Mutations in Indeterminate Thyroid Nodules: Systematic Review and Meta-analysis . Otolaryngology–Head and Neck Surgery 156:. https://doi.org/10.1177/0194599816685697
Angell TE (2017) RAS- positive thyroid nodules. Current Opinion in Endocrinology, Diabetes and Obesity 24
Marotta V, Bifulco M, Vitale M (2021) Significance of ras mutations in thyroid benign nodules and non‐medullary thyroid cancer. Cancers (Basel) 13
Xing M (2016) Clinical utility of RAS mutations in thyroid cancer: A blurred picture now emerging clearer. BMC Medicine 14
Liu R, Xing M (2016) TERT promoter mutations in thyroid cancer. Endocrine-Related Cancer 23:R143–R155
Acknowledgements
Sydney D. Finkelstein, MD, Chief Scientific Officer at Interpace Diagnostics, is acknowledged for his contribution to the molecular data and details of ThyGeNEXT and ThyraMIR test characteristics.
Author information
Authors and Affiliations
Contributions
Nicole A. Cipriani: conceptualization, methodology, formal analysis, investigation, writing—original draft, visualization Daniel N. Johnson: conceptualization, methodology, investigation, writing—original draft David H. Sarne: investigation, writing—review and editing Peter Angelos: investigation, writing—review and editing Ward Reeves: investigation, resources Tatjana Antic: conceptualization, writing—review and editing.
Corresponding author
Ethics declarations
Ethics Approval
Approval from the University of Chicago Internal Review Board was obtained.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cipriani, N.A., Johnson, D.N., Sarne, D.H. et al. The Significance of RAS-Like Mutations and MicroRNA Profiling in Predicting Malignancy in Thyroid Biopsy Specimens. Endocr Pathol 33, 446–456 (2022). https://doi.org/10.1007/s12022-022-09734-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-022-09734-0