Abstract
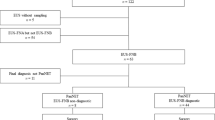
Neuroendocrine pancreatic tumors (PanNETs) are graded on the basis of their proliferative activity. Cytological samples are commonly the only samples available, but the determination of Ki-67 in cytology and its reliability as a measure of tumor mitotic activity is not well settled. We have retrospectively reviewed all the cases of FNA under EUS control of PanNETs in a 10-year period (2006–2016) in the Hospital Clínico San Carlos (Madrid). We identified 10 PanNET cases with histological correlation. Median age was 49.4 years and the patients were mainly women. PanNETs were located more frequently in the tail of the pancreas, with a median size of 33.8 mm. None of our cases was a grade 3 tumor. The seven grade 1 tumors confirmed in histology had consistent Ki-67 in cytology. In three cases (30 %), there were discrepancies between the Ki-67 index measured in cytology and histology, and the differences ranged from 2 to 15 %; all these cases were grade 2 tumors in histology and were graded as grade 1 tumors in FNA material. Our results are consistent with previous studies which showed understaging when tumor grade was assessed in cytological samples, mainly in G2 tumors. Previous literature has shown that Ki-67 assessment in EUS-FNA samples is a useful tool to rule out G3 tumors, but can be problematic for distinguishing G1 and G2 tumors.


Similar content being viewed by others
References
Farrell JM, Pang JC, Kim GE, Tabatabai ZL (2014) Pancreatic neuroendocrine tumors: accurate grading with Ki-67 index on fine-needle aspiration specimens using the WHO 2010/ENETS criteria. Cancer Cytopathol 122:770–8.
Hasegawa T, Yamao K, Hijioka S, Bhatia V, Mizuno N, Hara K, et al. (2014) Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy 46:32–8.
Larghi A, Capurso G, Carnuccio A, Ricci R, Alfieri S, Galasso D, et al. (2012) Ki-67 grading of nonfunctioning pancreatic neuroendocrine tumors on histologic samples obtained by EUS-guided fine-needle tissue acquisition: a prospective study. Gastrointest Endosc 76:570–7.
Weynand B, Borbath I, Bernard V, Sempoux C, Gigot J-F, Hubert C, et al. (2014) Pancreatic neuroendocrine tumour grading on endoscopic ultrasound-guided fine needle aspiration: high reproducibility and inter-observer agreement of the Ki-67 labelling index. Cytopathology 25:389–95.
McCall CM, Shi C, Cornish TC, Klimstra DS, Tang LH, Basturk O, et al. (2013) Grading of well-differentiated pancreatic neuroendocrine tumors is improved by the inclusion of both Ki67 proliferative index and mitotic rate. Am J Surg Pathol 37:1671–7.
Asa SL (2011) Pancreatic endocrine tumors. Mod Pathol. 24 Suppl 2:S66–77.
Klimstra DS, Modlin IR, Coppola D, Lloyd R V, Suster S (2010) The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 39:707–12.
Adsay V. Ki67 labeling index in neuroendocrine tumors of the gastrointestinal and pancreatobiliary tract: to count or not to count is not the question, but rather how to count (2012) Am J Surg Pathol 36:1743–6.
Piani C, Franchi GM, Cappelletti C, Scavini M, Albarello L, Zerbi A, et al. (2008) Cytological Ki-67 in pancreatic endocrine tumours: an opportunity for pre-operative grading. Endocr Relat Cancer 15:175–81.
Unno J, Kanno A, Masamune A, Kasajima A, Fujishima F, Ishida K, et al. (2014) The usefulness of endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of pancreatic neuroendocrine tumors based on the World Health Organization classification. Scand J Gastroenterol 49:1367–74.
Miller HC, Drymousis P, Flora R, Goldin R, Spalding D, Frilling A (2014) Role of Ki-67 proliferation index in the assessment of patients with neuroendocrine neoplasias regarding the stage of disease. World J Surg 38:1353–61.
Sugimoto M, Takagi T, Hikichi T, Suzuki R, Watanabe K, Nakamura J, et al. (2015) Efficacy of endoscopic ultrasonography-guided fine needle aspiration for pancreatic neuroendocrine tumor grading. World J Gastroenterol 21:8118–24.
Chatzipantelis P, Konstantinou P, Kaklamanos M, Apostolou G, Salla C (2009) The role of cytomorphology and proliferative activity in predicting biologic behavior of pancreatic neuroendocrine tumors: a study by endoscopic ultrasound-guided fine-needle aspiration cytology. Cancer 117:211–6.
Alexiev BA, Darwin PE, Goloubeva O, Ioffe OB (2009) Proliferative rate in endoscopic ultrasound fine-needle aspiration of pancreatic endocrine tumors: correlation with clinical behavior. Cancer 117:40–5.
Hooper K, Mukhtar F, Li S, Eltoum IA (2013) Diagnostic error assessment and associated harm of endoscopic ultrasound-guided fine-needle aspiration of neuroendocrine neoplasms of the pancreas. Cancer Cytopathol 121:653–60.
Tang LH, Gonen M, Hedvat C, Modlin IM, Klimstra DS (2012) Objective quantification of the Ki67 proliferative index in neuroendocrine tumors of the gastroenteropancreatic system: a comparison of digital image analysis with manual methods. Am J Surg Pathol 36:1761–70.
Hedvat C V (2010) Digital microscopy: past, present, and future. Arch Pathol Lab Med 134:1666–70.
Lewis RB, Lattin GE, Paal E (2010) Pancreatic endocrine tumors: radiologic-clinicopathologic correlation. Radiographics 30:1445–64.
Yang Z, Tang LH, Klimstra DS (2011) Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. Am J Surg Pathol 35:853–60.
Pinhel IF, Macneill FA, Hills MJ, Salter J, Detre S, A’hern R, et al. (2010) Extreme loss of immunoreactive p-Akt and p-Erk1/2 during routine fixation of primary breast cancer. Breast Cancer Res 12:R76.
Chatzipantelis P, Salla C, Konstantinou P, Karoumpalis I, Sakellariou S, Doumani I (2008) Endoscopic ultrasound-guided fine-needle aspiration cytology of pancreatic neuroendocrine tumors: a study of 48 cases. Cancer 114:255–62.
Gu M, Ghafari S, Lin F, Ramzy I (2005) Cytological diagnosis of endocrine tumors of the pancreas by endoscopic ultrasound-guided fine-needle aspiration biopsy. Diagn Cytopathol 32:204–10.
Chang F, Vu C, Chandra A, Meenan J, Herbert A (2006) Endoscopic ultrasound-guided fine needle aspiration cytology of pancreatic neuroendocrine tumours: cytomorphological and immunocytochemical evaluation. Cytopathology 17:10–7.
Kulke MH, Shah MH, Benson AB, Bergsland E, Berlin JD, Blaszkowsky LS, et al. (2015) Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw 13:78–108.
Pavel M, Baudin E, Couvelard A, Krenning E, Öberg K, Steinmüller T, et al. (2012) ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 95:157–76.
Costa FP, Gumz B, Pasche B (2012) Selecting patients for cytotoxic therapies in gastroenteropancreatic neuroendocrine tumours. Best Pract Res Clin Gastroenterol 26:843–54.
Artale S, Giannetta L, Cerea G, Maggioni D, Pedrazzoli P, Schiavetto I, et al. (2005) Treatment of metastatic neuroendocrine carcinomas based on WHO classification. Anticancer Res 25:4463–9.
Falconi M, Plockinger U, Kwekkeboom DJ, Manfredi R, Korner M, Kvols L, et al. (2006) Well-differentiated pancreatic nonfunctioning tumors/carcinoma. Neuroendocrinology 84:196–211.
Metz DC, Jensen RT (2008) Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology 135:1469–92.
Oberg K, Akerström G, Rindi G, Jelic S (2010) Neuroendocrine gastroenteropancreatic tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 21 Suppl 5:v223–7.
Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, et al. (2013) Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol 24:152–60.
Fasanella KE, McGrath KM, Sanders M, Brody D, Domsic R, Khalid A (2009) Pancreatic endocrine tumor EUS-guided FNA DNA microsatellite loss and mortality. Gastrointest Endosc 69:1074–80.
Acknowledgments
Special thanks are due to J. Miguel Esteban López-Jamar, Department of Gastroenterology, Hospital Clínico San Carlos, Madrid.
Authors’ Contribution
Cristina Díaz del Arco was responsible for the study conception and design, acquisition of data, analysis and interpretation of data, and drafting of the manuscript; J. Ángel Díaz Pérez for the acquisition of data, drafting of the manuscript, and critical revision; Luis Ortega Medina for study conception and design, analysis and interpretation of data, and critical revision; Javier Sastre Valera for the acquisition of data, analysis and interpretation of data, and critical revision; and M. Jesús Fernández Aceñero for the study conception and design, analysis and interpretation of data, and critical revision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
No funding was received for this report.
Informed Consent
Retrospective study: For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Díaz del Arco, C., Díaz Pérez, J.Á., Ortega Medina, L. et al. Reliability of Ki-67 Determination in FNA Samples for Grading Pancreatic Neuroendocrine Tumors. Endocr Pathol 27, 276–283 (2016). https://doi.org/10.1007/s12022-016-9455-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-016-9455-2