Abstract
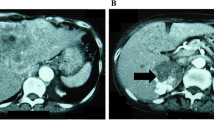
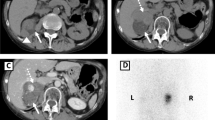
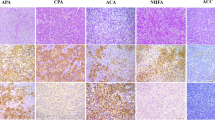
Most adrenocortical carcinomas (ACCs) produce excessive amounts of steroid hormones including aldosterone, cortisol, and steroid precursors. However, aldosterone- and cortisol-producing cells in ACCs have not yet been immunohistochemically described. We present a case of ACC causing mild primary aldosteronism and subclinical Cushing’s syndrome. Removal of the tumor cured both conditions. In order to examine the expression patterns of the steroidogenic enzymes responsible for adrenocortical hormone production, 10 tumor portions were immunohistochemically analyzed for aldosterone synthase (CYP11B2), 11β-hydroxylase (CYP11B1, cortisol-synthesizing enzyme), 3β-hydroxysteroid dehydrogenase (3βHSD, upstream enzyme for both CYP11B2 and CYP11B1), and 17α-hydroxylase/C17-20 lyase (CYP17, upstream enzyme for CYP11B1, but not for CYP11B1). CYP11B2, CYP11B1, and 3βHSD were expressed sporadically, and their expression patterns varied significantly among the different tumor portions examined. The expression of these enzymes was random and not associated with each other. CYP17 was expressed throughout the tumor, even in CYP11B2-positive cells. Small tumor cell populations were aldosterone- or cortisol-producing cells, as judged by 3βHSD coinciding with either CYP11B2 or CYP11B1, respectively. These results suggest that the tumor produced limited amounts of aldosterone and cortisol due to the lack of the coordinated expression of steroidogenic enzymes, which led to mild clinical expression in this case. We delineated the expression patterns of steroidogenic enzymes in ACC. The coordinated expression of steroidogenic enzymes in normal and adenoma cells was disturbed in ACC cells, resulting in the inefficient production of steroid hormones in relation to the large tumor volume.









Similar content being viewed by others
References
Else T, Kim AC, Sabolch A, et al. Adrenocortical carcinoma. Endocr Rev 35: 282–326, 2014.
Nishimoto K, Nakagawa K, Li D, et al. Adrenocortical zonation in humans under normal and pathological conditions. The Journal of clinical endocrinology and metabolism 95: 2296–2305, 2010.
Hattangady NG, Olala LO, Bollag WB, Rainey WE. Acute and chronic regulation of aldosterone production. Molecular and cellular endocrinology 350: 151–162, 2012.
Gomez-Sanchez CE, Qi X, Velarde-Miranda C, et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Molecular and cellular endocrinology 383: 111–117, 2014.
Nishimoto K, Tomlins SA, Kuick R, et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proceedings of the National Academy of Sciences of the United States of America 112: E4591-4599, 2015.
Ruifrok AC, Johnston DA Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol 23: 291–299, 2001.
Akehi Y, Kawate H, Murase K, et al. Proposed diagnostic criteria for subclinical Cushing’s syndrome associated with adrenal incidentaloma. Endocr J 60: 903–912, 2013.
Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. The Journal of clinical endocrinology and metabolism 93: 3266–3281, 2008.
Lau SK, Weiss LM. The Weiss system for evaluating adrenocortical neoplasms: 25 years later. Human pathology 40: 757–768, 2009.
Nishimoto K, Seki T, Kurihara I, et al. Case Report: Nodule Development From Subcapsular Aldosterone-Producing Cell Clusters Causes Hyperaldosteronism. The Journal of clinical endocrinology and metabolism 101: 6–9, 2016
Sasano H, Suzuki T, Nagura H, Nishikawa T. Steroidogenesis in human adrenocortical carcinoma: biochemical activities, immunohistochemistry, and in situ hybridization of steroidogenic enzymes and histopathologic study in nine cases. Human pathology 24: 397–404, 1993.
Sasano H, Miyazaki S, Sawai T, et al. Primary pigmented nodular adrenocortical disease (PPNAD): immunohistochemical and in situ hybridization analysis of steroidogenic enzymes in eight cases. Mod Pathol 5: 23–29, 1992.
Arlt W, Biehl M, Taylor AE, et al. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. The Journal of clinical endocrinology and metabolism 96: 3775–3784, 2011.
Nakamura Y, Maekawa T, Felizola SJ, et al. Adrenal CYP11B1/2 expression in primary aldosteronism: immunohistochemical analysis using novel monoclonal antibodies. Molecular and cellular endocrinology 392: 73–79, 2014.
Acknowledgments
We thank Dr. Takeshi Yamazaki at Hiroshima University for providing us with the anti-3βHSD antibody; Mr. Shinya Sasai at Tachikawa Hospital for his technical assistance with immunohistochemistry; as well as funding support from the Japan Society for the Promotion of Science (KAKENHI-Grants to T.U [#23791043], K.N [#26893261], and KM [#26461387]), the Suzuken Memorial Foundation (to KN), Yamaguchi Endocrine Research Foundation (to KN), Okinaka Memorial Institute for Medical Research (to KN), Federation of National Public Service Personnel Mutual Aid Associations (to KN), NIH HL27255 (to CEG-S), and Initiative for Rare and Undiagnosed Diseases (IRUD) by AMED (to YK).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
None declared.
Additional information
Toyoyoshi Uchida and Koshiro Nishimoto contributed equally to this work.
Rights and permissions
About this article
Cite this article
Uchida, T., Nishimoto, K., Fukumura, Y. et al. Disorganized Steroidogenesis in Adrenocortical Carcinoma, a Case Study. Endocr Pathol 28, 27–35 (2017). https://doi.org/10.1007/s12022-016-9441-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-016-9441-8