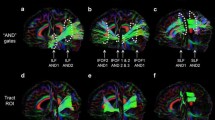
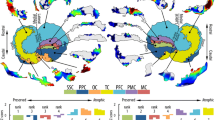
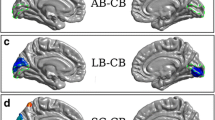
Abstract
Blindness represents a unique model to study how visual experience may shape the development of brain organization. Exploring how the structure of the corpus callosum (CC) reorganizes ensuing visual deprivation is of particular interest due to its important functional implication in vision (e.g., via the splenium of the CC). Moreover, comparing early versus late visually deprived individuals has the potential to unravel the existence of a sensitive period for reshaping the CC structure. Here, we develop a novel framework to capture a complete set of shape differences in the CC between congenitally blind (CB), late blind (LB) and sighted control (SC) groups. The CCs were manually segmented from T1-weighted brain MRI and modeled by 3D tetrahedral meshes. We statistically compared the combination of local area and thickness at each point between subject groups. Differences in area are found using surface tensor-based morphometry; thickness is estimated by tracing the streamlines in the volumetric harmonic field. Group differences were assessed on this combined measure using Hotelling’s T 2 test. Interestingly, we observed that the total callosal volume did not differ between the groups. However, our fine-grained analysis reveals significant differences mostly localized around the splenium areas between both blind groups and the sighted group (general effects of blindness) and, importantly, specific dissimilarities between the LB and CB groups, illustrating the existence of a sensitive period for reorganization. The new multivariate statistics also gave better effect sizes for detecting morphometric differences, relative to other statistics. They may boost statistical power for CC morphometric analyses.







Similar content being viewed by others
References
Adamson, C. L., Wood, A. G., Chen, J., Barton, S., Reutens, D. C., Pantelis, C., et al. (2011). Thickness profile generation for the corpus callosum using Laplace’s equation. Human Brain Mapping, 32(12), 2131–2140. doi:10.1002/hbm.21174.
Amedi, A., Raz, N., Pianka, P., Malach, R., & Zohary, E. (2003). Early ‘visual’ cortex activation correlates with superior verbal memory performance in the blind. Nature Neuroscience, 6(7), 758–766. doi:10.1038/nn1072.
Arsigny, V., Fillard, P., Pennec, X., & Ayache, N. (2006). Log-Euclidean metrics for fast and simple calculus on diffusion tensors. Magnetic Resonance in Medicine, 56(2), 411–421.
Bakircioglu, M., Joshi, S., & Miller, M. I. (1999). Landmark matching on brain surfaces via large deformation diffeomorphisms on the sphere. Proceedings of SPIE Medical Imaging, 3661, 710–715.
Bavelier, D., & Neville, H. J. (2002). Cross-modal plasticity: where and how? Nature Reviews Neuroscience, 3(6), 443–452. doi:10.1038/nrn848.
Bedny, M., Pascual-Leone, A., Dodell-Feder, D., Fedorenko, E., & Saxe, R. (2011). Language processing in the occipital cortex of congenitally blind adults. Proceedings of the National Academy of Sciences of the United States of America, 108(11), 4429–4434. doi:10.1073/pnas.1014818108.
Belluck, P. (2013). Device offers partial vision for the blind. The New York Times.
Bock, A. S., & Olavarria, J. F. (2011). Neonatal enucleation during a critical period reduces the precision of cortico-cortical projections in visual cortex. Neuroscience Letters, 501(3), 152–156. doi:10.1016/j.neulet.2011.07.005.
Bock, A. S., Olavarria, J. F., Leigland, L. A., Taber, E. N., Jespersen, S. N., & Kroenke, C. D. (2010). Diffusion tensor imaging detects early cerebral cortex abnormalities in neuronal architecture induced by bilateral neonatal enucleation: an experimental model in the ferret. Frontiers in Systems Neuroscience, 4, 149. doi:10.3389/fnsys.2010.00149.
Bock, A. S., Kroenke, C. D., Taber, E. N., & Olavarria, J. F. (2012). Retinal input influences the size and corticocortical connectivity of visual cortex during postnatal development in the ferret. Journal of Comparative Neurology, 520(5), 914–932. doi:10.1002/cne.22738.
Bock, A. S., Saenz, M., Tungaraza, R., Boynton, G. M., Bridge, H., & Fine, I. (2013). Visual callosal topography in the absence of retinal input. NeuroImage, 81, 325–334. doi:10.1016/j.neuroimage.2013.05.038.
Bridge, H., Cowey, A., Ragge, N., & Watkins, K. (2009). Imaging studies in congenital anophthalmia reveal preservation of brain architecture in ‘visual’ cortex. Brain, 132(Pt 12), 3467–3480. doi:10.1093/brain/awp279.
Caleo, M., Innocenti, G. M., & Ptito, M. (2013). Physiology and plasticity of interhemispheric connections. Neural Plasticity, 2013, 176183. doi:10.1155/2013/176183.
Chan, K. C., Cheng, J. S., Fan, S., Zhou, I. Y., Yang, J., & Wu, E. X. (2012). In vivo evaluation of retinal and callosal projections in early postnatal development and plasticity using manganese-enhanced MRI and diffusion tensor imaging. NeuroImage, 59(3), 2274–2283. doi:10.1016/j.neuroimage.2011.09.055.
Chung, M. K. (2012). Computational neuroanatomy: The methods. World Scientific Publishing Company.
Chung, M. K., Dalton, K. M., & Davidson, R. J. (2008). Tensor-based cortical surface morphometry via weighted spherical harmonic representation. IEEE Transactions on Medical Imaging, 27(8), 1143–1151.
Collignon, O., Voss, P., Lassonde, M., & Lepore, F. (2009). Cross-modal plasticity for the spatial processing of sounds in visually deprived subjects. Experimental Brain Research, 192(3), 343–358. doi:10.1007/s00221-008-1553-z.
Collignon, O., Champoux, F., Voss, P., & Lepore, F. (2011a). Sensory rehabilitation in the plastic brain. Progress in Brain Research, 191, 211–231. doi:10.1016/B978-0-444-53752-2.00003-5.
Collignon, O., Vandewalle, G., Voss, P., Albouy, G., Charbonneau, G., Lassonde, M., et al. (2011b). Functional specialization for auditory-spatial processing in the occipital cortex of congenitally blind humans. Proceedings of the National Academy of Sciences of the United States of America, 108(11), 4435–4440. doi:10.1073/pnas.1013928108.
Collignon, O., Dormal, G., Albouy, G., Vandewalle, G., Voss, P., Phillips, C., et al. (2013a). Impact of blindness onset on the functional organization and the connectivity of the occipital cortex. Brain, 136(Pt 9), 2769–2783. doi:10.1093/brain/awt176.
Collignon, O., Dormal, G., & Lepore, F. (2013b). Building the brain in the dark: Functional and specific crossmodal reorganization in the occipital cortex of blind individuals. In M. Jenkin, J. Steeves, & L. Harris (Eds.), Plasticity in sensory systems. Cambridge: Cambridge University Press.
Colom, R., Stein, J. L., Rajagopalan, L., Martínez, K., Hermel, D., Wang, Y., et al. (2013). Hippocampal structure and human cognition: key role of spatial processing and evidence supporting the efficiency hypothesis in females. Intelligence, in press.
Davatzikos, C. (1996). Spatial normalization of 3D brain images using deformable models. Journal of Computer Assisted Tomography , 20(4), 656–665.
Davatzikos, C., Vaillant, M., Resnick, S. M., Prince, J. L., Letovsky, S., & Bryan, R. N. (1996). A computerized approach for morphological analysis of the corpus callosum. Journal of Computer Assisted Tomography, 20(1), 88–97.
Di Paola, M., Luders, E., Cherubini, A., Sanchez-Castaneda, C., Thompson, P. M., Toga, A. W., et al. (2012). Multimodal MRI analysis of the corpus callosum reveals white matter differences in presymptomatic and early Huntington’s disease. Cerebral Cortex, 22(12), 2858–2866. doi:10.1093/cercor/bhr360.
Dormal, G., Lepore, F., & Collignon, O. (2012). Plasticity of the dorsal “spatial” stream in visually deprived individuals. Neural Plasticity, 2012, 687659. doi:10.1155/2012/687659.
Dougherty, R. F., Ben-Shachar, M., Bammer, R., Brewer, A. A., & Wandell, B. A. (2005). Functional organization of human occipital-callosal fiber tracts. Proceedings of the National Academy of Sciences of the United States of America, 102(20), 7350–7355. doi:10.1073/pnas.0500003102.
Elad, M., Milanfar, P., & Golub, G. H. (2004). Shape from moments - an estimation theory perspective. Transactions on Signal Processing , 52(7), 1814–1829. doi:10.1109/tsp.2004.828919.
Fischl, B., Sereno, M. I., & Dale, A. M. (1999). Cortical surface-based analysis II: inflation, flattening, and a surface-based coordinate system. NeuroImage, 9(2), 195–207.
Fish, S. E., Rhoades, R. W., Bennett-Clarke, C. A., Figley, B., & Mooney, R. D. (1991). Organization, development and enucleation-induced alterations in the visual callosal projection of the hamster: single axon tracing with phaseolus vulgaris leucoagglutinin and Di-I. European Journal of Neuroscience, 3(12), 1255–1270.
Gu, X., Wang, Y., Chan, T. F., Thompson, P. M., & Yau, S.-T. (2004). Genus zero surface conformal mapping and its application to brain surface mapping. IEEE Transactions on Medical Imaging, 23(8), 949–958.
He, Q., Duan, Y., Yin, X., Gu, X., Karsch, K., & Miles, J. (2009) Shape analysis of corpus callosum in autism subtype using planar conformal mapping. SPIE, Medical Imaging, 7262. doi:10.1117/12.812285.
Heimler, B., Weisz, N., Collignon, O. (2014) Revisiting the adaptive and maladaptive effects of crossmodal plasticity. Neuroscience, 283, 44–63.
Herron, T. J., Kang, X., & Woods, D. L. (2012). Automated measurement of the human corpus callosum using MRI. Frontiers in Neuroinformatics , 6, 25. doi:10.3389/fninf.2012.00025.
Hofer, S., & Frahm, J. (2006). Topography of the human corpus callosum revisited–comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage, 32(3), 989–994. doi:10.1016/j.neuroimage.2006.05.044.
Hua, X., Leow, A. D., Levitt, J. G., Caplan, R., Thompson, P. M., & Toga, A. W. (2009). Detecting brain growth patterns in normal children using tensor-based morphometry. Human Brain Mapping, 30(1), 209–219. doi:10.1002/hbm.20498.
Jenkinson, M., & Smith, S. (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5(2), 143–156.
Jiang, J., Zhu, W., Shi, F., Liu, Y., Li, J., Qin, W., et al. (2009). Thick visual cortex in the early blind. Journal of Neuroscience, 29(7), 2205–2211. doi:10.1523/JNEUROSCI. 5451-08.2009.
Jones, S. E., Buchbinder, B. R., & Aharon, I. (2000). Three-dimensional mapping of cortical thickness using Laplace’s equation. Human Brain Mapping, 11(1), 12–32.
Joshi, S. H., Narr, K. L., Philips, O. R., Nuechterlein, K. H., Asarnow, R. F., Toga, A. W., et al. (2013). Statistical shape analysis of the corpus callosum in Schizophrenia. NeuroImage, 64, 547–559. doi:10.1016/j.neuroimage.2012.09.024.
Lederman, C., Joshi, A., Dinov, I., Vese, L., Toga, A., & Van Horn, J. D. (2011). The generation of tetrahedral mesh models for neuroanatomical MRI. NeuroImage, 55(1), 153–164. doi:10.1016/j.neuroimage.2010.11.013.
Leporé, N., Brun, C., Chou, Y.-Y., Chiang, M.-C., Dutton, R. A., Hayashi, K. M., et al. (2008). Generalized tensor-based morphometry of HIV/AIDS using multivariate statistics on deformation tensors. IEEE Transactions on Medical Imaging, 27(1), 129–141.
Leporé, N., Shi, Y., Lepore, F., Fortin, M., Voss, P., Chou, Y. Y., et al. (2009). Pattern of hippocampal shape and volume differences in blind subjects. NeuroImage, 46(4), 949–957. doi:10.1016/j.neuroimage.2009.01.071.
Leporé, N., Voss, P., Lepore, F., Chou, Y. Y., Fortin, M., Gougoux, F., et al. (2010). Brain structure changes visualized in early- and late-onset blind subjects. NeuroImage, 49(1), 134–140. doi:10.1016/j.neuroimage.2009.07.048.
Levin, N., Dumoulin, S. O., Winawer, J., Dougherty, R. F., & Wandell, B. A. (2010). Cortical maps and white matter tracts following long period of visual deprivation and retinal image restoration. Neuron, 65(1), 21–31. doi:10.1016/j.neuron.2009.12.006.
Li, X., Guo, X., Wang, H., He, Y., Gu, X., & Qin, H. (2007). Harmonic volumetric mapping for solid modeling applications. Paper presented at the Proceedings of the 2007 ACM symposium on Solid and physical modeling, Beijing, China.
Li, B., Li, X., Wang, K., & Qin, H. (2013). Surface mesh to volumetric spline conversion with generalized polycubes. IEEE Transactions on Visualization and Computer Graphics, 19(9), 1539–1551. doi:10.1109/tvcg.2012.177.
Lombaert, H., Grady, L., Polimeni, J. R., & Cheriet, F. (2013). FOCUSR: feature oriented correspondence using spectral regularization - a method for accurate surface matching. IEEE Transactions on Pattern Analysis and Machine Intelligence, 35(9), 2143–2160.
Luders, E., Narr, K. L., Zaidel, E., Thompson, P. M., Jancke, L., & Toga, A. W. (2006). Parasagittal asymmetries of the corpus callosum. Cerebral Cortex, 16(3), 346–354. doi:10.1093/cercor/bhi112.
Luders, E., Thompson, P. M., & Toga, A. W. (2010). The development of the corpus callosum in the healthy human brain. Journal of Neuroscience, 30(33), 10985–10990. doi:10.1523/JNEUROSCI. 5122-09.2010.
Merabet, L. B., Rizzo, J. F., Amedi, A., Somers, D. C., & Pascual-Leone, A. (2005). What blindness can tell us about seeing again: merging neuroplasticity and neuroprostheses. Nature Reviews Neuroscience, 6(1), 71–77. doi:10.1038/nrn1586.
Merabet, L. B., Rizzo, J. F., 3rd, Pascual-Leone, A., & Fernandez, E. (2007). ‘Who is the ideal candidate?’: decisions and issues relating to visual neuroprosthesis development, patient testing and neuroplasticity. Journal of Neural Engineering, 4(1), S130–S135. doi:10.1088/1741-2560/4/1/S15.
Monje, M., Thomason, M. E., Rigolo, L., Wang, Y., Waber, D. P., Sallan, S. E., et al. (2013). Functional and structural differences in the hippocampus associated with memory deficits in adult survivors of acute lymphoblastic leukemia. Pediatric Blood & Cancer, 60(2), 293–300. doi:10.1002/pbc.24263.
Noppeney, U. (2007). The effects of visual deprivation on functional and structural organization of the human brain. Neuroscience and Biobehavioral Reviews, 31(8), 1169–1180. doi:10.1016/j.neubiorev.2007.04.012.
Noppeney, U., Friston, K. J., Ashburner, J., Frackowiak, R., & Price, C. J. (2005). Early visual deprivation induces structural plasticity in gray and white matter. Current Biology, 15(13), R488–R490. doi:10.1016/j.cub.2005.06.053.
Olavarria, J. F., & Van Sluyters, R. C. (1995). Overall pattern of callosal connections in visual cortex of normal and enucleated cats. Journal of Comparative Neurology, 363(2), 161–176. doi:10.1002/cne.903630202.
Olavarria, J., Malach, R., & Van Sluyters, R. C. (1987). Development of visual callosal connections in neonatally enucleated rats. Journal of Comparative Neurology, 260(3), 321–348. doi:10.1002/cne.902600302.
Pai, D., Soltanian-Zadeh, H., & Hua, J. (2011). Evaluation of fiber bundles across subjects through brain mapping and registration of diffusion tensor data. NeuroImage, 54(Suppl 1), S165–S175. doi:10.1016/j.neuroimage.2010.05.085.
Paillé, G.-P., & Poulin, P. (2012). SMI 2012: full as-conformal-as-possible discrete volumetric mapping. Computers and Graphics, 36(5), 427–433. doi:10.1016/j.cag.2012.03.014.
Pan, W. J., Wu, G., Li, C. X., Lin, F., Sun, J., & Lei, H. (2007). Progressive atrophy in the optic pathway and visual cortex of early blind Chinese adults: a voxel-based morphometry magnetic resonance imaging study. NeuroImage, 37(1), 212–220. doi:10.1016/j.neuroimage.2007.05.014.
Pandya, D. N., Karol, E. A., & Heilbronn, D. (1971). The topographical distribution of interhemispheric projections in the corpus callosum of the rhesus monkey. Brain Research, 32(1), 31–43.
Park, H. J., Jeong, S. O., Kim, E. Y., Kim, J. I., Park, H., Oh, M. K., et al. (2007). Reorganization of neural circuits in the blind on diffusion direction analysis. Neuroreport, 18(17), 1757–1760. doi:10.1097/WNR.0b013e3282f13e66.
Park, H. J., Lee, J. D., Kim, E. Y., Park, B., Oh, M. K., Lee, S., et al. (2009). Morphological alterations in the congenital blind based on the analysis of cortical thickness and surface area. NeuroImage, 47(1), 98–106. doi:10.1016/j.neuroimage.2009.03.076.
Ptito, M., Schneider, F. C., Paulson, O. B., & Kupers, R. (2008). Alterations of the visual pathways in congenital blindness. Experimental Brain Research, 187(1), 41–49. doi:10.1007/s00221-008-1273-4.
Qin, W., Liu, Y., Jiang, T., & Yu, C. (2013). The development of visual areas depends differently on visual experience. PloS One, 8(1), e53784. doi:10.1371/journal.pone.0053784.
Qiu, A., Bitouk, D., & Miller, M. I. (2006a). Smooth functional and structural maps on the neocortex via orthonormal bases of the Laplace-Beltrami operator. IEEE Transactions on Medical Imaging, 25(10), 1296–1306.
Qiu, A., Rosenau, B. J., Greenberg, A. S., Hurdal, M. K., Barta, P., Yantis, S., et al. (2006b). Estimating linear cortical magnification in human primary visual cortex via dynamic programming. NeuroImage, 31(1), 125–138. doi:10.1016/j.neuroimage.2005.11.049.
Ricciardi, E., & Pietrini, P. (2011). New light from the dark: what blindness can teach us about brain function. Current Opinion in Neurology, 24(4), 357–363. doi:10.1097/WCO.0b013e328348bdbf.
Shi, Y., Lai, R., Morra, J. H., Dinov, I., Thompson, P. M., & Toga, A. W. (2010). Robust surface reconstruction via Laplace-Beltrami eigen-projection and boundary deformation. IEEE Transactions on Medical Imaging, 29(12), 2009–2022. doi:10.1109/TMI.2010.2057441.
Shi, J., Thompson, P. M., Gutman, B., & Wang, Y. (2013a). Surface fluid registration of conformal representation: application to detect disease burden and genetic influence on hippocampus. NeuroImage, 78C, 111–134. doi:10.1016/j.neuroimage.2013.04.018.
Shi, J., Wang, Y., Ceschin, R., An, X., Lao, Y., Vanderbilt, D., et al. (2013b). A multivariate surface-based analysis of the putamen in premature newborns: regional differences within the ventral striatum. PloS One, 8(7), e66736. doi:10.1371/journal.pone.0066736.
Shi, R., Zeng, W., Su, Z., Damasio, H., Lu, Z., Wang, Y., et al. (2013c, June) Hyperbolic Harmonic Mapping for Constrained Brain Registration. In IEEE Conf. Comp. Vis. Patt. Recog. CVPR’13.
Shi, J., Lepore, N., Gutman, B. A., Thompson, P. M., Baxter, L. C., Caselli, R. L., et al. (2014). Genetic influence of apolipoprotein E4 genotype on hippocampal morphometry: an N = 725 surface-based Alzheimer’s disease neuroimaging initiative study. Human Brain Mapping. doi:10.1002/hbm.22447.
Shimony, J. S., Burton, H., Epstein, A. A., McLaren, D. G., Sun, S. W., & Snyder, A. Z. (2006). Diffusion tensor imaging reveals white matter reorganization in early blind humans. Cerebral Cortex, 16(11), 1653–1661. doi:10.1093/cercor/bhj102.
Shu, N., Li, J., Li, K., Yu, C., & Jiang, T. (2009). Abnormal diffusion of cerebral white matter in early blindness. Human Brain Mapping, 30(1), 220–227. doi:10.1002/hbm.20507.
Sieving, P. A., Caruso, R. C., Tao, W., Coleman, H. R., Thompson, D. J. S., Fullmer, K. R., et al. (2006). Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proceedings of the National Academy of Sciences of the United States of America, 103(10), 3896–3901. doi:10.1073/pnas.0600236103.
Steele, C. J., Bailey, J. A., Zatorre, R. J., & Penhune, V. B. (2013). Early musical training and white-matter plasticity in the corpus callosum: evidence for a sensitive period. Journal of Neuroscience, 33(3), 1282–1290. doi:10.1523/JNEUROSCI. 3578-12.2013.
Styner, M., Lieberman, J. A., McClure, R. K., Weinberger, D. R., Jones, D. W., & Gerig, G. (2005a). Morphometric analysis of lateral ventricles in schizophrenia and healthy controls regarding genetic and disease-specific factors. Proceedings of the National Academy of Sciences of the United States of America, 102(13), 4872–4877.
Styner, M. A., Oguz, I., Smith, R. G., Cascio, C., & Jomier, M. (2005b). Corpus callosum subdivision based on a probabilistic model of inter-hemispheric connectivity. Medical Image Computing and Computer-Assisted Intervention, 8(Pt 2), 765–772.
Tan, Y., Hua, J., & Qin, H. (2010). Physically based modeling and simulation with dynamic spherical volumetric simplex splines. Computer-Aided Design, 42(2), 95–108. doi:10.1016/j.cad.2009.02.014.
Tepest, R., Jacobi, E., Gawronski, A., Krug, B., Moller-Hartmann, W., Lehnhardt, F. G., et al. (2010). Corpus callosum size in adults with high-functioning autism and the relevance of gender. Psychiatry Research, 183(1), 38–43. doi:10.1016/j.pscychresns.2010.04.007.
Thompson, P. M., Giedd, J. N., Woods, R. P., MacDonald, D., Evans, A. C., & Toga, A. W. (2000). Growth patterns in the developing human brain detected using continuum-mechanical tensor mapping. Nature, 404(6774), 190–193.
Thompson, P. M., Narr, K. L., Blanton, R. E., & Toga, A. W. (2003). Mapping structural alterations of the corpus callosum during brain development and degeneration. In E. Zaidel & M. Iacobini (Eds.), The parallel brain: The cognitive neuroscience of the corpus callosum (pp. 93–130). Cambridge: The MIT Press.
Thompson, P. M., Hayashi, K. M., de Zubicaray, G. I., Janke, A. L., Rose, S. E., Semple, J., et al. (2004a). Mapping hippocampal and ventricular change in Alzheimer’s disease. NeuroImage, 22(4), 1754–1766.
Thompson, P. M., Hayashi, K. M., Sowell, E. R., Gogtay, N., Giedd, J. N., Rapoport, J. L., et al. (2004b). Mapping cortical change in Alzheimer's disease, brain development, and schizophrenia. NeuroImage, 23(Supplement 1), S2–S18.
Thompson, P. M., Dutton, R. A., Hayashi, K. M., Lu, A., Lee, S. E., Lee, J. Y., et al. (2006). 3D mapping of ventricular and corpus callosum abnormalities in HIV/AIDS. NeuroImage, 31(1), 12–23. doi:10.1016/j.neuroimage.2005.11.043.
Tomaiuolo, F., Campana, S., Collins, D. L., Fonov, V. S., Ricciardi, E., Sartori, G., et al. (2014). Morphometric changes of the corpus callosum in congenital blindness. PloS One, 9(9), e107871. doi:10.1371/journal.pone.0107871.
Veraart, C., Duret, F., Brelen, M., Oozeer, M., & Delbeke, J. (2004). Vision rehabilitation in the case of blindness. Expert Review of Medical Devices, 1(1), 139–153. doi:10.1586/17434440.1.1.139.
Vidal, C. N., Nicolson, R., DeVito, T. J., Hayashi, K. M., Geaga, J. A., Drost, D. J., et al. (2006). Mapping corpus callosum deficits in autism: an index of aberrant cortical connectivity. Biological Psychiatry, 60(3), 218–225. doi:10.1016/j.biopsych.2005.11.011.
Voss, P., & Zatorre, R. J. (2012). Occipital cortical thickness predicts performance on pitch and musical tasks in blind individuals. Cerebral Cortex, 22(11), 2455–2465. doi:10.1093/cercor/bhr311.
Voss, P., Fortin, M., Corbo, V., Pruessner, J. C., & Lepore, F. (2013). Assessment of the caudate nucleus and its relation to route learning in both congenital and late blind individuals. BMC Neuroscience, 14, 113. doi:10.1186/1471-2202-14-113.
Wang, Y., Gu, X., Chan, T. F., Thompson, P. M., & Yau, S.-T. (2004a). Volumetric harmonic brain mapping. Paper presented at the Biomedical Imaging: Nano to Macro, 2004. IEEE International Symposium on.
Wang, Y., Gu, X., & Yau, S.-T. (2004b). Volumetric harmonic map. Communications in Information and Systems, 3(3), 191–202.
Wang, Y., Zhang, J., Gutman, B., Chan, T. F., Becker, J. T., Aizenstein, H. J., et al. (2010). Multivariate tensor-based morphometry on surfaces: application to mapping ventricular abnormalities in HIV/AIDS. NeuroImage, 49(3), 2141–2157. doi:10.1016/j.neuroimage.2009.10.086.
Wang, Y., Song, Y., Rajagopalan, P., An, T., Liu, K., Chou, Y. Y., et al. (2011). Surface-based TBM boosts power to detect disease effects on the brain: an N = 804 ADNI study. NeuroImage, 56(4), 1993–2010. doi:10.1016/j.neuroimage.2011.03.040.
Wang, K., Li, X., Li, B., Xu, H., & Qin, H. (2012a). Restricted trivariate polycube splines for volumetric data modeling. IEEE Transactions on Visualization and Computer Graphics, 18(5), 703–716. doi:10.1109/TVCG.2011.102.
Wang, Y., Panigrahy, A., Shi, J., Ceschin, R., Nie, Z., Nelson, M. D., et al. (2012b) 3D vs. 2D surface shape analysis of the corpus collosum in premature neonates. In MICCAI: Workshop on Paediatric and Perinatal Imaging, Nice, France.
Wang, Y., Shi, J., Yin, X., Gu, X., Chan, T. F., Yau, S.-T., et al. (2012c). Brain surface conformal parameterization with the Ricci flow. IEEE Transactions on Medical Imaging, 31(2), 251–264.
Wang, D., Qin, W., Liu, Y., Zhang, Y., Jiang, T., & Yu, C. (2013a). Altered white matter integrity in the congenital and late blind people. Neural Plasticity, 2013, 128236. doi:10.1155/2013/128236.
Wang, G., Li, J., Chen, J., Wang, L., Jiang, Z., Su, Q., et al. (2013b). A heat kernel based brain grey matter morphometry system. Paper presented at the 3rd MICCAI Workshop on Multimodal Brain Image Analysis (MBIA).
Wang, Y., Yuan, L., Shi, J., Greve, A., Ye, J., Toga, A. W., et al. (2013c). Applying tensor-based morphometry to parametric surfaces can improve MRI-based disease diagnosis. NeuroImage, 74, 209–230. doi:10.1016/j.neuroimage.2013.02.011.
Wüstefeld, T. (2010). bilderzucht - blog. http://www.bilderzucht.de/blog/3d-pixel-voxel.
Xu, L. (2013). Combining thickness information with surface tensor-based morphometry for the 3D statistical analysis of the corpus callosum. Master Thesis, Arizona State University, Tempte, AZ.
Xu, H., Yu, W., Gu, S., & Li, X. (2013a). Biharmonic volumetric mapping using fundamental solutions. IEEE Transactions on Visualization and Computer Graphics, 19(5), 787–798. doi:10.1109/TVCG.2012.173.
Xu, L., Collignon, O., Wang, G., Kang, Y., Leporé, F., Shi, J., et al. (2013b). Combining Thickness Information with Surface Tensor-based Morphometry for the 3D Statistical Analysis of the Corpus Callosum. Paper presented at the 4th MICCAI Workshop on Mathematical Foundations of Computational Anatomy (MFCA).
Yeo, B. T., Sabuncu, M. R., Vercauteren, T., Ayache, N., Fischl, B., & Golland, P. (2010). Spherical demons: fast diffeomorphic landmark-free surface registration. IEEE Transactions on Medical Imaging, 29(3), 650–668. doi:10.1109/TMI.2009.2030797.
Yu, C., Shu, N., Li, J., Qin, W., Jiang, T., & Li, K. (2007). Plasticity of the corticospinal tract in early blindness revealed by quantitative analysis of fractional anisotropy based on diffusion tensor tractography. NeuroImage, 36(2), 411–417. doi:10.1016/j.neuroimage.2007.03.003.
Yushkevich, P. A., Piven, J., Hazlett, H. C., Smith, R. G., Ho, S., Gee, J. C., et al. (2006). User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage, 31(3), 1116–1128. doi:10.1016/j.neuroimage.2006.01.015.
Zatorre, R. J., Fields, R. D., & Johansen-Berg, H. (2012). Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nature Neuroscience, 15(4), 528–536. doi:10.1038/nn.3045.
Acknowledgments
This work was funded by the National Institute on Aging (R21AG043760 to LX, JS and YW), the National Institute for Biomedical Imaging and Bioengineering (R21EB012177 to YL and NL), the Arizona Alzheimer’s Consortium (ADHS14-052688 to YW), the National Science Foundation (DMS-1413417, IIS-1421165 to YW), the joint special fund of Shandong province Natural Science Fundation (ZR2013FL008 to GW) and the European Research Council starting grant MADVIS (ERC-StG 337573 to OC). YW is also supported, in part, by U54 EB020403 (the “Big Data to Knowledge” Program), supported by the National Cancer Institute (NCI), the NIBIB and a cross-NIH Consortium.
Author information
Authors and Affiliations
Corresponding author
Additional information
Natasha Leporé and Yalin Wang are equal senior authors.
Rights and permissions
About this article
Cite this article
Shi, J., Collignon, O., Xu, L. et al. Impact of Early and Late Visual Deprivation on the Structure of the Corpus Callosum: A Study Combining Thickness Profile with Surface Tensor-Based Morphometry. Neuroinform 13, 321–336 (2015). https://doi.org/10.1007/s12021-014-9259-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12021-014-9259-9