Abstract
Objective
We examined how the sex steroids influence the synthesis of gonadotropins.
Materials and Methods
The effects of sex steroids estradiol (E2), progesterone (P4), and dihydrotestosterone (DHT) in pituitary gonadotroph cell model (LβT2 cells) in vitro and ovary-intact rats in vivo were examined. The effects of sex steroids on Kiss1 gene expression in the hypothalamus were also examined in ovary-intact rats.
Results
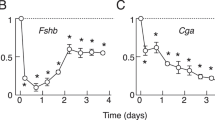
In LβT2 cells, E2 increased common glycoprotein alpha (Cga) and luteinizing hormone beta (Lhb) subunit promoter activity as well as their mRNA expression. Although gonadotropin subunit promoter activity was not modulated by P4, Cga and Lhb mRNA expression was increased by P4. DHT inhibited Cga and Lhb mRNA expression with a concomitant decrease in their promoter activity. During the 2-week administration of exogenous E2 to ovary-intact rats, the estrous cycle determined by vaginal smears was disrupted. P4 or DHT administration completely eliminated the estrous cycle. Protein expression of all three gonadotropin subunits within the pituitary gland was inhibited by E2 or P4 treatment in vivo; however, DHT reduced Cga expression but did not modulate Lhb or follicle-stimulating hormone beta subunit expression. E2 administration significantly repressed Kiss1 mRNA expression in a posterior hypothalamic region that included the arcuate nucleus. P4 and DHT did not modulate Kiss1 mRNA expression in this region. In contrast, P4 administration significantly inhibited Kiss1 mRNA expression in the anterior region of the hypothalamus that included the anteroventral periventricular nucleus. The expression of gonadotropin-releasing hormone (Gnrh) mRNA in the anterior hypothalamic region, where the preoptic area is located, appeared to be decreased by treatment with E2 and P4.
Conclusion
Our findings suggest that sex steroids have different effects in the hypothalamus and pituitary gland.








Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during this study are available from the corresponding authors on reasonable request.
References
S.D. Gharib, M.E. Wierman, M.A. Shupnik, W.W. Chin, Molecular-Biology of the Pituitary Gonadotropins. Endocrine Rev 11(1), 177–199 (1990). https://doi.org/10.1210/edrv-11-1-177
K. Skorupskaite, J.T. George, R.A. Anderson, The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update 20(4), 485–500 (2014). https://doi.org/10.1093/humupd/dmu009
S. Adachi, S. Yamada, Y. Takatsu, H. Matsui, M. Kinoshita, K. Takase, H. Sugiura, T. Ohtaki, H. Matsumoto, Y. Uenoyama, H. Tsukamura, K. Inoue, K. Maeda, Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 53(2), 367–378 (2007). https://doi.org/10.1262/jrd.18146
J.T. Smith, M.J. Cunningham, E.F. Rissman, D.K. Clifton, R.A. Steiner, Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146(9), 3686–3692 (2005). https://doi.org/10.1210/en.2005-0488
J.T. Smith, H.M. Dungan, E.A. Stoll, M.L. Gottsch, R.E. Braun, S.M. Eacker, D.K. Clifton, R.A. Steiner, Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146(7), 2976–2984 (2005). https://doi.org/10.1210/en.2005-0323
J.T. Smith, C.M. Clay, A. Caraty, I.J. Clarke, KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 148(3), 1150–1157 (2007). https://doi.org/10.1210/en.2006-1435
J.D. Blaustein, The year in neuroendocrinology. Mol Endocrinol 24(1), 252–260 (2010). https://doi.org/10.1210/me.2009-0350
M.A. Shupnik, S.D. Gharib, W.W. Chin, Divergent effects of estradiol on gonadotropin gene transcription in pituitary fragments. Mol Endocrinol 3(3), 474–480 (1989). https://doi.org/10.1210/mend-3-3-474
C.L. Phillips, L.W. Lin, J.C. Wu, K. Guzman, A. Milsted, W.L. Miller, 17 Beta-estradiol and progesterone inhibit transcription of the genes encoding the subunits of ovine follicle-stimulating hormone. Mol Endocrinol 2(7), 641–649 (1988). https://doi.org/10.1210/mend-2-7-641
J.S. Finkelstein, L.S. O’Dea, R.W. Whitcomb, W.F. Crowley Jr, Sex steroid control of gonadotropin secretion in the human male. II. Effects of estradiol administration in normal and gonadotropin-releasing hormone-deficient men. J Clin Endocrinol Metab 73(3), 621–628 (1991). https://doi.org/10.1210/jcem-73-3-621
J. Clarkson, A.E. Herbison, Oestrogen, kisspeptin, GPR54 and the pre-ovulatory luteinising hormone surge. J Neuroendocrinol 21(4), 305–311 (2009). https://doi.org/10.1111/j.1365-2826.2009.01835.x
M.R. Soules, R.A. Steiner, D.K. Clifton, N.L. Cohen, S. Aksel, W.J. Bremner, Progesterone modulation of pulsatile luteinizing hormone secretion in normal women. J Clin Endocrinol Metab 58(2), 378–383 (1984). https://doi.org/10.1210/jcem-58-2-378
R.L. Goodman, E.L. Bittman, D.L. Foster, F.J. Karsch, The endocrine basis of the synergistic suppression of luteinizing hormone by estradiol and progesterone. Endocrinology 109(5), 1414–1417 (1981). https://doi.org/10.1210/endo-109-5-1414
L.A. Nolan, A. Levy, The effects of testosterone and oestrogen on gonadectomised and intact male rat anterior pituitary mitotic and apoptotic activity. J Endocrinol 188(3), 387–396 (2006). https://doi.org/10.1677/joe.1.06508
U.B. Kaiser, E. Sabbagh, R.A. Katzenellenbogen, P.M. Conn, W.W. Chin, A mechanism for the differential regulation of gonadotropin subunit gene expression by gonadotropin-releasing hormone. Proc Natl Acad Sci USA 92(26), 12280–12284 (1995)
W. Kuohung, M. Burnett, D. Mukhtyar, E. Schuman, J. Ni, W.F. Crowley, M.A. Glicksman, U.B. Kaiser, A high-throughput small-molecule ligand screen targeted to agonists and antagonists of the G-protein-coupled receptor GPR54. J Biomol Screen 15(5), 508–517 (2010). https://doi.org/10.1177/1087057110369701
Y.L. Son, T. Ubuka, R.P. Millar, H. Kanasaki, K. Tsutsui, Gonadotropin-inhibitory hormone inhibits GnRH-induced gonadotropin subunit gene transcriptions by inhibiting AC/cAMP/PKA-dependent ERK pathway in LbetaT2 cells. Endocrinology 153(5), 2332–2343 (2012). https://doi.org/10.1210/en.2011-1904
Y. Yamada, H. Yamamoto, T. Yonehara, H. Kanasaki, H. Nakanishi, E. Miyamoto, K. Miyazaki, Differential activation of the luteinizing hormone beta-subunit promoter by activin and gonadotropin-releasing hormone: a role for the mitogen-activated protein kinase signaling pathway in LbetaT2 gonadotrophs. Biol Reprod 70(1), 236–243 (2004). https://doi.org/10.1095/biolreprod.103.019588
T. Mijiddorj, H. Kanasaki, A. Oride, T. Hara, U. Sukhbaatar, T. Tumurbaatar, S. Kyo, Interaction between kisspeptin and adenylate cyclase-activating polypeptide 1 on the expression of pituitary gonadotropin subunits: a study using mouse pituitary lbetaT2 cells. Biol Reprod 96(5), 1043–1051 (2017). https://doi.org/10.1093/biolre/iox030
H. Kanasaki, M. Tselmeg, A. Oride, U. Sukhbaatar, T. Hara, S. Kyo, Pulsatile kisspeptin effectively stimulates gonadotropin-releasing hormone (GnRH)-producing neurons. Gynecol Endocrinol 33(9), 721–727 (2017). https://doi.org/10.1080/09513590.2017.1318277
T. Mijiddorj, H. Kanasaki, U. Sukhbaatar, A. Oride, T. Hara, S. Kyo, Mutual regulation by GnRH and kisspeptin of their receptor expression and its impact on the gene expression of gonadotropin subunits. Gen Comp Endocrinol 246, 382–389 (2017). https://doi.org/10.1016/j.ygcen.2017.01.014
M.L. Wong, J.F. Medrano, Real-time PCR for mRNA quantitation. BioTechniques 39(1), 75–85 (2005)
S.A. Bustin, V. Benes, T. Nolan, M.W. Pfaffl, Quantitative real-time RT-PCR-a perspective. J Mol Endocrinol 34(3), 597–601 (2005). https://doi.org/10.1677/jme.1.01755
T.J. Spady, R. Shayya, V.G. Thackray, L. Ehrensberger, J.S. Bailey, P.L. Mellon, Androgen regulates follicle-stimulating hormone beta gene expression in an activin-dependent manner in immortalized gonadotropes. Mol Endocrinol 18(4), 925–940 (2004). https://doi.org/10.1210/me.2003-0115
V.G. Thackray, S.M. McGillivray, P.L. Mellon, Androgens, progestins, and glucocorticoids induce follicle-stimulating hormone beta-subunit gene expression at the level of the gonadotrope. Mol Endocrinol 20(9), 2062–2079 (2006). https://doi.org/10.1210/me.2005-0316
H. Kanasaki, I.N. Purwana, T. Mijiddorj, U. Sukhbaatar, A. Oride, K. Miyazaki, Effects of estradiol and progesterone on gonadotropin LHbeta- and FSHbeta-subunit promoter activities in gonadotroph LbetaT2 cells. Neuro Endocrinol Lett 33(6), 608–613 (2012)
Q. Xie, Y. Kang, C. Zhang, Y. Xie, C. Wang, J. Liu, C. Yu, H. Zhao, D. Huang, The role of kisspeptin in the control of the hypothalamic-pituitary-gonadal axis and reproduction. Front Endocrinol (Lausanne) 13, 925206 (2022). https://doi.org/10.3389/fendo.2022.925206
F. Filippi, M. Reschini, E. Polledri, A. Cecchele, C. Guarneri, P. Vigano, S. Fustinoni, P. Platteau, E. Somigliana, Progestin-primed ovarian stimulation for fertility preservation in women with cancer: a comparative study. PLoS One 18(3), e0280238 (2023). https://doi.org/10.1371/journal.pone.0280238
T.M. Plant, Hypothalamic control of the pituitary-gonadal axis in higher primates: key advances over the last two decades. J Neuroendocrinol 20(6), 719–726 (2008). https://doi.org/10.1111/j.1365-2826.2008.01708.x
J.C. Marshall, A.C. Dalkin, D.J. Haisenleder, M.L. Griffin, R.P. Kelch, GnRH pulses-the regulators of human reproduction. Trans Am Clin Climatol Assoc 104, 31–46 (1993)
D.J. Bernard, J. Fortin, Y. Wang, P. Lamba, Mechanisms of FSH synthesis: what we know, what we don’t, and why you should care. Fertil Steril 93(8), 2465–2485 (2010). https://doi.org/10.1016/j.fertnstert.2010.03.034
M. Schubert, L. Perez Lanuza, J. Gromoll, Pharmacogenetics of FSH Action in the Male. Front Endocrinol (Lausanne) 10, 47 (2019). https://doi.org/10.3389/fendo.2019.00047
Acknowledgements
The manuscript was edited by ThinkSCIENCE, Inc.
Funding
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to H.K. and A.O.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yacca, S.S., Kanasaki, H., Tumurbaatar, T. et al. Changes in pituitary gonadotropin subunits and hypothalamic Kiss-1 gene expression by administration of sex steroids in ovary-intact female rats. Endocrine 83, 733–746 (2024). https://doi.org/10.1007/s12020-023-03596-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03596-0