Abstract
Purpose
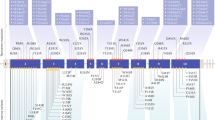
Multiple endocrine neoplasia type 4 (MEN4) is a rare multiglandular endocrine neoplasia syndrome, associated with a wide tumor spectrum but hallmarked by primary hyperparathyroidism, which represents the most common clinical feature, followed by pituitary (functional and non-functional) adenomas, and neuroendocrine tumors. MEN4 clinically overlaps MEN type 1 (MEN1) but differs from it for milder clinical features and an older patient’s age at onset. The underlying mutated gene, CDKN1B, encodes the cell cycle regulator p27, implicated in cellular proliferation, motility and apoptosis. Given the paucity of MEN4 cases described in the literature, possible genotype–phenotype correlations have not been thoroughly assessed, and specific clinical recommendations are lacking. The present review provides an extensive overview of molecular genetics and clinical features of MEN4, with the aim of contributing to delineate peculiar strategies for clinical management, screening and follow-up of the last and least known MEN syndrome.
Methods
A literature search was performed through online databases like MEDLINE and Scopus.
Conclusions
MEN4 is much less common that MEN1, tend to present later in life with a more indolent course, although involving the same primary organs as MEN1. As a consequence, MEN4 patients might need specific diagnostic and therapeutic approaches and a different strategy for screening and follow-up. Further studies are needed to assess the real oncological risk of MEN4 carriers, and to establish a standardized screening protocol. Furthermore, a deeper understanding of molecular genetics of MEN4 is needed in order to explore p27 as a novel therapeutic target.


Similar content being viewed by others
References
M.L. Brandi, S.K. Agarwal, N.D. Perrier, K.E. Lines, G.D. Valk, R.V. Thakker, Multiple Endocrine Neoplasia Type 1: latest insights. Endocr. Rev. 42(2), 133–70. (2021)
M.L. Brandi, R.F. Gagel, A. Angeli, J.P. Bilezikian, P. Beck-Peccoz, C. Bordi et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J. Clin. Endocrinol. Metab. 86(12), 5658–5671 (2001)
A. Fritz, A. Walch, K. Piotrowska, M. Rosemann, E. Schaffer, K. Weber et al. Recessive transmission of a multiple endocrine neoplasia syndrome in the rat. Cancer Res. 62(11), 3048–3051 (2002)
N.S. Pellegata, L. Quintanilla-Martinez, H. Siggelkow, E. Samson, K. Bink, H. Hofler et al. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc. Natl. Acad. Sci. USA 103(42), 15558–15563 (2006)
M. Georgitsi, A. Raitila, A. Karhu, R.B. van der Luijt, C.M. Aalfs, T. Sane et al. Germline CDKN1B/p27Kip1 mutation in multiple endocrine neoplasia. J. Clin. Endocrinol. Metab. 92(8), 3321–3325 (2007)
R. Halperin, L. Arnon, S. Nasirov, L. Friedensohn, M. Gershinsky, A. Telerman, Germline CDKN1B variant type and site are associated with phenotype in MEN4. Endocr. Relat. Cancer 30(1), e220174 (2022)
H. Singeisen, M.M. Renzulli, V. Pavlicek, P. Probst, F. Hauswirth, M.K. Muller, Multiple endocrine neoplasia type 4: a new member of the MEN family. Endocr. Connect. 12(2), e220411 (2023)
S.F. Razavipour, K.B. Harikumar, J.M. Slingerland, p27 as a transcriptional regulator: new roles in development and cancer. Cancer Res. 80(17), 3451–3458 (2020)
D. Bencivenga, I. Caldarelli, E. Stampone, F.P. Mancini, M.L. Balestrieri, F. Della Ragione et al. p27(Kip1) and human cancers: a reappraisal of a still enigmatic protein. Cancer Lett. 403, 354–65. (2017)
H. Li, M. Collado, A. Villasante, A. Matheu, C.J. Lynch, M. Canamero et al. p27(Kip1) directly represses Sox2 during embryonic stem cell differentiation. Cell Stem Cell 11, 845–852 (2012)
G. Baldassarre, B. Belletti, M.S. Nicoloso, M. Schiappacassi, A. Vecchione, P. Spessotto et al. p27(Kip1)-stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell 7, 51–63 (2005)
C.I. Rubin, G.F. Atweh, The role of stathmin in the regulation of the cell cycle. J. Cell Biochem. 93, 242–250 (2004)
R.T. Perchey, M.P. Serres, A. Nowosad, J. Creff, C. Callot, A. Gay et al. p27(Kip1) regulates the microtubule bundling activity of PRC1. Biochim. Biophys. Acta Mol. Cell Res. 1865, 1630–1639 (2018)
M.M. Babu, The contribution of intrinsically disordered regions to protein function, cellular complexity, and human disease. Biochem. Soc. Trans. 44(5), 1185–200. (2016)
A. Nowosad, A. Besson, CDKN1B/p27 regulates autophagy via the control of Ragulator and MTOR activity in amino acid-deprived cells. Autophagy 16(12), 2297–2298 (2020)
O.V. Bochis, A. Irimie, M. Pichler, I. Berindan-Neagoe, The role of Skp2 and its substrate CDKN1B (p27) in colorectal cancer. J. Gastrointestin Liver Dis. 24(2), 225–234 (2015)
S. Dietrich, J. Hullein, S.C. Lee, B. Hutter, D. Gonzalez, S. Jayne et al. Recurrent CDKN1B (p27) mutations in hairy cell leukemia. Blood 126(8), 1005–1008 (2015)
D. Viotto, F. Russo, I. Anania, I. Segatto, G.L. Rampioni Vinciguerra, A. Dall’Acqua et al. CDKN1B mutation and copy number variation are associated with tumor aggressiveness in luminal breast cancer. J. Pathol. 253(2), 234–45. (2021)
C.E. Barbieri, S.C. Baca, M.S. Lawrence, F. Demichelis, M. Blattner, J.P. Theurillat et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat. Genet. 44(6), 685–689 (2012)
J.M. Francis, A. Kiezun, A.H. Ramos, S. Serra, C.S. Pedamallu, Z.R. Qian et al. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nat. Genet. 45(12), 1483–1486 (2013)
C.S. Martins, R.C. Camargo, F.P. Saggioro, L. Neder, H.R. Machado, A.C. Moreira et al. P27/CDKN1B translational regulators in pituitary tumorigenesis. Horm. Metab. Res. 48(12), 840–846 (2016)
B. Belletti, G. Baldassarre, Roles of CDKN1B in cancer? Aging (Albany NY) 7(8), 529–530 (2015)
R.V. Thakker, Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4). Mol. Cell Endocrinol. 386(1-2), 2–15 (2014)
S.K. Karnik, C.M. Hughes, X. Gu, O. Rozenblatt-Rosen, G.W. McLean, Y. Xiong et al. Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. Proc. Natl. Acad. Sci. USA 102(41), 14659–14664 (2005)
J. Huang, B. Gurung, B. Wan, S. Matkar, N.A. Veniaminova, K. Wan et al. The same pocket in menin binds both MLL and JUND but has opposite effects on transcription. Nature 482(7386), 542–546 (2012)
S. Borsari, E. Pardi, N.S. Pellegata, M. Lee, F. Saponaro, L. Torregrossa et al. Loss of p27 expression is associated with MEN1 gene mutations in sporadic parathyroid adenomas. Endocrine 55(2), 386–97. (2017)
R. Alrezk, F. Hannah-Shmouni, C.A. Stratakis, MEN4 and CDKN1B mutations: the latest of the MEN syndromes. Endocr. Relat. Cancer 24(10), T195–T208 (2017)
T.A. Milne, C.M. Hughes, R. Lloyd, Z. Yang, O. Rozenblatt-Rosen, Y. Dou et al. Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc. Natl. Acad. Sci. USA 102(3), 749–754 (2005)
S.K. Agarwal, C.M. Mateo, S.J. Marx, Rare germline mutations in cyclin-dependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. J. Clin. Endocrinol. Metab. 94(5), 1826–1834 (2009)
S. Molatore, I. Marinoni, M. Lee, E. Pulz, M.R. Ambrosio, E.C. degli Uberti et al. A novel germline CDKN1B mutation causing multiple endocrine tumors: clinical, genetic and functional characterization. Hum. Mutat. 31(11), E1825–E1835 (2010)
M.C. Lemos, R.V. Thakker, Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum. Mutat. 29(1), 22–32 (2008)
P. Concolino, A. Costella, E. Capoluongo, Multiple endocrine neoplasia type 1 (MEN1): an update of 208 new germline variants reported in the last nine years. Cancer Genet. 209(1-2), 36–41 (2016)
S.A. Wells Jr., S.L. Asa, H. Dralle, R. Elisei, D.B. Evans, R.F. Gagel et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 25(6), 567–610 (2015)
I. Christakis, W. Qiu, S.M. Hyde, G.J. Cote, E.G. Grubbs, N.D. Perrier et al. Genotype-phenotype pancreatic neuroendocrine tumor relationship in multiple endocrine neoplasia type 1 patients: a 23-year experience at a single institution. Surgery 163(1), 212–217 (2018)
S.Y. Li, Y.Q. Ding, Y.L. Si, M.J. Ye, C.M. Xu, X.P. Qi, 5P strategies for management of multiple endocrine neoplasia Type 2: a paradigm of precision medicine. Front. Endocrinol. (Lausanne) 11, 543246 (2020)
D. Muller, K. Thieke, A. Burgin, A. Dickmanns, M. Eilers, Cyclin E-mediated elimination of p27 requires its interaction with the nuclear pore-associated protein mNPAP60. EMBO J. 19(10), 2168–2180 (2000)
A. Sakurai, S. Suzuki, S. Kosugi, T. Okamoto, S. Uchino, A. Miya et al. Multiple endocrine neoplasia type 1 in Japan: establishment and analysis of a multicentre database. Clin. Endocrinol. (Oxf.) 76(4), 533–539 (2012)
E. Tham, U. Grandell, E. Lindgren, G. Toss, B. Skogseid, M. Nordenskjold, Clinical testing for mutations in the MEN1 gene in Sweden: a report on 200 unrelated cases. J. Clin. Endocrinol. Metab. 92(9), 3389–3395 (2007)
R.A. Carvalho, B. Urtremari, A.A.L. Jorge, L.S. Santana, E.P.S. Quedas, T. Sekiya et al. Germline mutation landscape of multiple endocrine neoplasia type 1 using full gene next-generation sequencing. Eur. J. Endocrinol. 179(6), 391–407 (2018)
S.K. Agarwal, The future: genetics advances in MEN1 therapeutic approaches and management strategies. Endocr. Relat. Cancer 24(10), T119–T34. (2017)
J.J. Turner, P.T. Christie, S.H. Pearce, P.D. Turnpenny, R.V. Thakker, Diagnostic challenges due to phenocopies: lessons from multiple Endocrine Neoplasia type1 (MEN1). Hum. Mutat. 31(1), E1089–E1101 (2010)
L.B. Nachtigall, F.J. Guarda, K.E. Lines, A. Ghajar, L. Dichtel, G. Mumbach et al. Clinical MEN-1 Among a Large Cohort of Patients With Acromegaly. J. Clin. Endocrinol. Metab. 105(6), e2271–e2281 (2020)
J.R. Burgess, B. Nord, R. David, T.M. Greenaway, V. Parameswaran, C. Larsson et al. Phenotype and phenocopy: the relationship between genotype and clinical phenotype in a single large family with multiple endocrine neoplasia type 1 (MEN 1). Clin. Endocrinol. (Oxf.) 53(2), 205–211 (2000)
P.J. Newey, R.V. Thakker, Role of multiple endocrine neoplasia type 1 mutational analysis in clinical practice. Endocr. Pract. 17(Suppl 3), 8–17 (2011)
R.W. Carroll, Multiple endocrine neoplasia type 1 (MEN1). Asia Pac. J. Clin. Oncol. 9(4), 297–309 (2013)
E.O. Mamedova, D.A. Dimitrova, Z.E. Belaya, G.A. Melnichenko,, The role of non-coding RNAs in the pathogenesis of multiple endocrine neoplasia syndrome type 1. Probl. Endokrinol. 66(2), 4–12 (2020).
E. Luzi, S. Ciuffi, F. Marini, C. Mavilia, G. Galli, M.L. Brandi, Analysis of differentially expressed microRNAs in MEN1 parathyroid adenomas. Am. J. Transl. Res. 9(4), 1743–53. (2017)
L.R. Yates, S. Knappskog, D. Wedge, J.H.R. Farmery, S. Gonzalez, I. Martincorena et al. Genomic evolution of breast cancer metastasis and relapse. Cancer Cell 32(2), 169–184 e7 (2017)
C.E. Barbieri, C.H. Bangma, A. Bjartell, J.W. Catto, Z. Culig, H. Gronberg et al. The mutational landscape of prostate cancer. Eur. Urol. 64(4), 567–576 (2013)
E. Lavezzi, A. Brunetti, V. Smiroldo, G. Nappo, V. Pedicini, E. Vitali et al. Case report: new CDKN1B mutation in multiple Endocrine Neoplasia Type 4 and brief literature review on clinical management. Front. Endocrinol. 13, 773143 (2022)
P. Goudet, P. Cougard, B. Verges, A. Murat, B. Carnaille, A. Calender et al. Hyperparathyroidism in multiple endocrine neoplasia type I: surgical trends and results of a 256-patient series from Groupe D’etude des Neoplasies Endocriniennes Multiples Study Group. World J. Surg. 25(7), 886–890 (2001)
M.S. Elston, G.Y. Meyer-Rochow, M. Dray, M. Swarbrick, J.V. Conaglen, Early onset primary Hyperparathyroidism Associated with a Novel Germline Mutation in CDKN1B. Case Rep. Endocrinol. 2015, 510985 (2015)
P. Goudet, A. Dalac, M. Le Bras, C. Cardot-Bauters, P. Niccoli, N. Levy-Bohbot et al. MEN1 disease occurring before 21 years old: a 160-patient cohort study from the Groupe d’etude des Tumeurs Endocrines. J. Clin. Endocrinol. Metab. 100(4), 1568–1577 (2015)
B. Verges, F. Boureille, P. Goudet, A. Murat, A. Beckers, G. Sassolas et al. Pituitary disease in MEN type 1 (MEN1): data from the France-Belgium MEN1 multicenter study. J. Clin. Endocrinol. Metab. 87(2), 457–465 (2002)
J.M. de Laat, O.M. Dekkers, C.R. Pieterman, W.P. Kluijfhout, A.R. Hermus, A.M. Pereira et al. Long-term natural course of pituitary tumors in patients with MEN1: results From the DutchMEN1 Study Group (DMSG). J. Clin. Endocrinol. Metab. 100(9), 3288–3296 (2015)
M.A. Tichomirowa, M. Lee, A. Barlier, A.F. Daly, I. Marinoni, M.L. Jaffrain-Rea et al. Cyclin-dependent kinase inhibitor 1B (CDKN1B) gene variants in AIP mutation-negative familial isolated pituitary adenoma kindreds. Endocr. Relat. Cancer 19(3), 233–241 (2012)
D. Donegan, N. Singh Ospina, R. Rodriguez-Gutierrez, Z. Al-Hilli, G.B. Thompson, B.L. Clarke et al. Long-term outcomes in patients with multiple endocrine neoplasia type 1 and pancreaticoduodenal neuroendocrine tumours. Clin. Endocrinol. 86(2), 199–206 (2017)
A. Frederiksen, M. Rossing, P. Hermann, C. Ejersted, R.V. Thakker, M. Frost, Clinical Features of Multiple Endocrine Neoplasia Type 4: Novel pathogenic variant and review of published cases. J. Clin. Endocrinol. Metab. 104(9), 3637–46. (2019)
R.V. Thakker, P.J. Newey, G.V. Walls, J. Bilezikian, H. Dralle, P.R. Ebeling et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J. Clin. Endocrinol. Metab. 97(9), 2990–3011 (2012)
H.R. Choi, S.H. Choi, S.M. Choi, J.K. Kim, C.R. Lee, S.W. Kang et al. Benefit of diverse surgical approach on short-term outcomes of MEN1-related hyperparathyroidism. Sci. Rep. 10(1), 10634 (2020)
F. Giusti, F. Tonelli, M.L. Brandi, Primary hyperparathyroidism in multiple endocrine neoplasia type 1: when to perform surgery. Clinics 67(Suppl 1), 141–144 (2012). Suppl 1
N. Aygun, M. Uludag, Surgical treatment of primary hyperparathyroidism: which therapy to whom? Sisli Etfal Hastan. Tip. Bul. 53(3), 201–14. (2019)
M.E. Molitch, Diagnosis and treatment of pituitary adenomas: a review. JAMA 317(5), 516–524 (2017)
S. Sambugaro, M. Di Ruvo, M.R. Ambrosio, N.S. Pellegata, M. Bellio, A. Guerra et al. Early onset acromegaly associated with a novel deletion in CDKN1B 5’UTR region. Endocrine 49(1), 58–64 (2015)
V. Andreasi, C. Ricci, S. Partelli, G. Guarneri, C. Ingaldi, F. Muffatti et al. Predictors of disease recurrence after curative surgery for nonfunctioning pancreatic neuroendocrine neoplasms (NF-PanNENs): a systematic review and meta-analysis. J. Endocrinol. Investig. 45(4), 705–18. (2022)
J.Y. Zhang, P.L. Kunz, Making sense of a complex disease: a practical approach to managing Neuroendocrine Tumors. JCO Oncol. Pract. 18(4), 258–64. (2022)
I.M. Chu, L. Hengst, J.M. Slingerland, The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat. Rev. Cancer 8(4), 253–267 (2008)
D. Busse, R.S. Doughty, T.T. Ramsey, W.E. Russell, J.O. Price, W.M. Flanagan et al. Reversible G(1) arrest induced by inhibition of the epidermal growth factor receptor tyrosine kinase requires up-regulation of p27(KIP1) independent of MAPK activity. J. Biol. Chem. 275(10), 6987–6995 (2000)
J. Liang, J.M. Slingerland, Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle 2(4), 339–345 (2003)
K. Szymonowicz, S. Oeck, N.M. Malewicz, V. Jendrossek, New Insights into Protein Kinase B/Akt Signaling: role of Localized Akt Activation and Compartment-Specific Target Proteins for the Cellular Radiation Response. Cancers (Basel) 10(3), 78 (2018)
K. Shanmugasundaram, K. Block, B.K. Nayak, C.B. Livi, M.A. Venkatachalam, S. Sudarshan, PI3K regulation of the SKP-2/p27 axis through mTORC2. Oncogene 32(16), 2027–2036 (2013)
Z. Hao, S. Huang, E3 ubiquitin ligase Skp2 as an attractive target in cancer therapy. Front. Biosci. (Landmark Ed.) 20(3), 474–490 (2015)
Acknowledgements
This study is part of the ‘Neuroendocrine Tumors Innovation Knowledge and Education’ project led by Prof. A.M.I, Prof. A.C and Prof. A.F which aims at increasing the knowledge on neuroendocrine tumors. We would like to acknowledge all the Collaborators of the “NIKE” project.
Author contributions
Each Author gave a substantial contribute to the paper and approved the final version to be published.
Funding
This work was not supported by any grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g., a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ruggeri, R.M., Benevento, E., De Cicco, F. et al. Multiple endocrine neoplasia type 4 (MEN4): a thorough update on the latest and least known men syndrome. Endocrine 82, 480–490 (2023). https://doi.org/10.1007/s12020-023-03497-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03497-2