Abstract
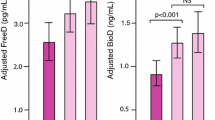
The 25 hydroxyvitamin D [25(OH)D] is the major metabolite for ascertaining vitamin D status, which circulates bound to a specific carrier (vitamin D-binding protein - VDBP). A portion that circulates unbound vary according to the VDBP genotype. This study evaluates the behavior of different forms of 25(OH)D, before and after supplementation with 14,000 IU of vitamin D3, weekly for 12 weeks, in individuals with primary hyperparathyroidism and controls. Fifty-six patients with active primary hyperparathyroidism (PHPT) and 64 paired controls (CTRL), not taking vitamin D3 for the last three months, were enrolled. The genetic isotypes of VDBP were determined to calculate bioavailable and free 25(OH)D. A p < 0.05 was considered significant. There were no statistical differences in free, bioavailable, and total 25(OH)D levels between PHPT and CTRL groups at baseline. The distribution of VDBP haplotypes 1s/1s, 1f/1f, 1s/1f, 2/2, 1s/2, and 1f/2 was similar between groups. After supplementation, all three forms of 25(OH)D proportionally increased within each group, although the percentage increment was lower in the PHPT group (p < 0.05). Total 25(OH)D is better correlated with PTH in the PHPT group than bioavailable and free 25(OH)D (r = −0.41; p < 0.05). The concentrations of total, free, and bioavailable 25(OH)D were similar in both PHPT and CTRL groups, and all forms increased proportionally after supplementation, although this increment percentage was higher in the CTRL group, with a subsequent reduction of PTH and AP. Total 25(OH)D correlated better with PTH than other forms, suggesting no advantages in measuring free or bioavailable 25(OH)D in these situations.



Similar content being viewed by others
Change history
15 February 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12020-023-03311-z
References
S.-W. Chang, H.-C. Lee, Vitamin D and health - The missing vitamin in humans. Pediatr. Neonatol. 60, 237–244 (2019)
M.L. Kottler, K.P. Schlingmann, Genetic diseases of vitamin D metabolizing enzymes. Endocrinology. (2017). https://www.endo.theclinics.com/article/S0889-8529(17)30073-7/abstract
J.A. Eisman, R.M. Shepard, H.F. DeLuca, Determination of 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 in human plasma using high-pressure liquid chromatography. Anal. Biochem. 80, 298–305 (1977)
F. Bienaimé, D. Prié, G. Friedlander, J.C. Souberbielle, Vitamin D metabolism and activity in the parathyroid gland. Mol. Cell Endocrinol. 347, 30–41 (2011)
J. Silver, J. Russell, L.M. Sherwood, Regulation by vitamin D metabolites of messenger ribonucleic acid for preproparathyroid hormone in isolated bovine parathyroid cells. Proc. Natl Acad. Sci. 82, 4270–4273 (1985)
D.D. Bikle, J. Schwartz, Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front Endocrinol. (2019). https://doi.org/10.3389/fendo.2019.00317
R. Takiar, P.L. Lutsey, D. Zhao, E. Guallar, A.L.C. Schneider, M.E. Grams et al. The associations of 25-hydroxyvitamin D levels, vitamin D binding protein gene polymorphisms, and race with risk of incident fracture-related hospitalization: Twenty-year follow-up in a bi-ethnic cohort (the ARIC Study). Bone 78, 94–101 (2015)
M.D. Walker, K.K. Nishiyama, B. Zhou, E. Cong, J. Wang, J.A. Lee et al. Effect of Low Vitamin D on Volumetric Bone Mineral Density, Bone Microarchitecture, and Stiffness in Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 101, 905–913 (2016)
P. Boudou, F. Ibrahim, C. Cormier, E. Sarfati, J.C. Souberbielle, A very high incidence of low 25 hydroxy-vitamin D serum concentration in a French population of patients with primary hyperparathyroidism. J. Endocrinol. Investig. 29, 511–515 (2006)
B. Moosgaard, P. Vestergaard, L. Heickendorff, F. Melsen, P. Christiansen, L. Mosekilde, Vitamin D status, seasonal variations, parathyroid adenoma weight and bone mineral density in primary hyperparathyroidism. Clin. Endocrinol. 63, 506–513 (2005)
D.S. Rao, M. Honasoge, G.W. Divine, E.R. Phillips, M.W. Lee, M.R. Ansari et al. Effect of vitamin D nutrition on parathyroid adenoma weight: pathogenetic and clinical implications. J. Clin. Endocrinol. Metab. 85, 1054–1058 (2000)
D.S. Rao, G. Agarwal, G.B. Talpos, E.R. Phillips, F. Bandeira, S.K. Mishra et al. Role of vitamin D and calcium nutrition in disease expression and parathyroid tumor growth in primary hyperparathyroidism: a global perspective. J. Bone Min. Res. 17, N75–N80 (2002)
B. Moosgaard, P. Vestergaard, L. Heickendorff, F. Melsen, P. Christiansen, L. Mosekilde, Plasma 25-hydroxyvitamin D and not 1,25-dihydroxyvitamin D is associated with parathyroid adenoma secretion in primary hyperparathyroidism: a cross-sectional study. Eur. J. Endocrinol. 155, 237–244 (2006)
S.S. Maeda, G.L. Saraiva, L.F. Hayashi, M.S. Cendoroglo, L.R. Ramos, M. Corrêa, P. de et al. Seasonal variation in the serum 25-hydroxyvitamin D levels of young and elderly active and inactive adults in São Paulo, Brazil: The São PAulo Vitamin D Evaluation Study (SPADES). Dermatoendocrinol 5, 211–217 (2013)
B.N. Fitzpatrick, Fitzpatrick tongue guard/retractors. Br. J. Oral. Surg. 16, 274 (1979)
C.M. Henderson, P.L. Lutsey, J.R. Misialek, T.J. Laha, E. Selvin, J.H. Eckfeldt et al. Measurement by a Novel LC-MS/MS Methodology Reveals Similar Serum Concentrations of Vitamin D-Binding Protein in Blacks and Whites. Clin. Chem. 62, 179–187 (2016)
M.R. Denburg, A.N. Hoofnagle, S. Sayed, J. Gupta, I.H. de Boer, L.J. Appel et al. Comparison of Two ELISA Methods and Mass Spectrometry for Measurement of Vitamin D-Binding Protein: Implications for the Assessment of Bioavailable Vitamin D Concentrations Across Genotypes. J. Bone Min. Res. 31, 1128–1136 (2016)
M.M.L. Kizys, M.G. Cardoso, S.C. Lindsey, M.Y. Harada, F.A. Soares, M.C.C. Melo et al. Optimizing nucleic acid extraction from thyroid fine-needle aspiration cells in stained slides, formalin-fixed/paraffin-embedded tissues, and long-term stored blood samples. Arq. Bras. Endocrinol. Metab. 56, 618–626 (2012)
J. Arnaud, J. Constans, Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP). Hum. Genet 92, 183–188 (1993)
D.D. Bikle, E. Gee, B. Halloran, M.A. Kowalski, E. Ryzen, J.G. Haddad, Assessment of the Free Fraction of 25-Hydroxyvitamin D in Serum and Its Regulation by Albumin and the Vitamin D-Binding Protein *. J Clin Endocrinol Metab. (1986). https://doi.org/10.1210/jcem-63-4-954
C. Battista, V. Guarnieri, V. Carnevale, F. Baorda, M. Pileri, M. Garrubba et al. Vitamin D status in primary hyperparathyroidism: effect of genetic background. Endocrine 55, 266–272 (2017)
L. Meng, S. Liu, A. Al-Dayyeni, Z. Sheng, Z. Zhou, X. Wang, Comparison of Initial Clinical Presentations between Primary Hyperparathyroidism Patients from New Brunswick and Changsha. Int J. Endocrinol. 2018, 6282687 (2018)
I. Bhan, Vitamin d binding protein and bone health. Int J. Endocrinol. 2014, 561214 (2014)
R. Bouillon, F. Schuit, L. Antonio, F. Rastinejad, Vitamin D Binding Protein: A Historic Overview. Front Endocrinol. 10, 910 (2019)
K.C. Norris, S.F. Williams, Race/Ethnicity, Serum 25-Hydroxyvitamin D, and Heart Disease. JAMA 310, 153–155 (2013)
B.R. Santos, N.C. Costa, T.R. Silva, K. Oppermann, J.A. Magalhães, G. Casanova et al. Prevalence of vitamin D deficiency in women from southern Brazil and association with vitamin D-binding protein levels and GC-DBP gene polymorphisms. PLoS One. 14, e0226215 (2019)
G. Viccica, F. Cetani, E. Vignali, M. Miccoli, C. Marcocci, Impact of vitamin D deficiency on the clinical and biochemical phenotype in women with sporadic primary hyperparathyroidism. Endocrine 55, 256–265 (2017)
W. Rankin, Primary hyperparathyroidism - is vitamin D supplementation safe? Aust. Fam. Physician. 40, 881–884 (2011)
V.N. Shah, C.S. Shah, S.K. Bhadada, D.S. Rao, Effect of 25 (OH) D replacements in patients with primary hyperparathyroidism (PHPT) and coexistent vitamin D deficiency on serum 25(OH) D, calcium and PTH levels: a meta-analysis and review of literature. Clin. Endocrinol. 80, 797–803 (2014)
X. Wang, S.A. Shapses, S. Wei, D. Sukumar, J. Ghosh, Vitamin D-binding protein levels in female patients with primary hyperparathyroidism. Endocr. Pr. 19, 609–613 (2013)
D.D. Bikle, B.P. Halloran, E. Gee, E. Ryzen, J.G. Haddad, Free 25-hydroxyvitamin D levels are normal in subjects with liver disease and reduced total 25-hydroxyvitamin D levels. J Clin Investig. 748–752 (1986). https://doi.org/10.1172/jci112636
L.C Pop, S.A. Shapses, B.Chang, W.Sun, X.Wang. Vitamin d-binding protein in healthy pre- and postmenopausal women: relationship with estradiol concentrations. Endocrine Practice. 2015 936–942 (2015). https://doi.org/10.4158/ep15623.or
M.R. Bennett, A. Pordal, C. Haffner, L. Pleasant, Q. Ma, P. Devarajan, Urinary Vitamin D-Binding Protein as a Biomarker of Steroid-Resistant Nephrotic Syndrome. Biomark. Insights. 11, 1–6 (2016)
X. Wang, S.A. Shapses, H. Al-Hraishawi, Free and bioavailable 25-hydroxyvitamin d levels in patients with primary hyperparathyroidism. Endocr. Pr. 23, 66–71 (2017)
X. Wang, Z.Sheng, L. Meng, C.Su, S.Trooskin, S.A. Shapses. 25-Hydroxyvitamin D and Vitamin D Binding Protein Levels in Patients With Primary Hyperparathyroidism Before and After Parathyroidectomy. Front. Endocrnol. 2019. https://doi.org/10.3389/fendo.2019.00171
L. Rolighed, L. Rejnmark, T. Sikjaer, L. Heickendorff, P. Vestergaard, L. Mosekilde et al. Vitamin D treatment in primary hyperparathyroidism: a randomized placebo controlled trial. J. Clin. Endocrinol. Metab. 99, 1072–1080 (2014)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest. Our article analyzes the usefulness of free 25(OH)D in individuals with primary hyperparathyroidism before and after supplementation with cholecalciferol. There are few studies in the literature on this topic, in addition we can contribute to clinical practice by verifying whether free 25(OH)D is necessary to evaluate the patient with primary hyperparathyroidism.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
dos Santos, L.M., Ohe, M.N., Pallone, S.G. et al. Levels of bioavailable, and free forms of 25(OH)D after supplementation with vitamin D3 in primary hyperparathyroidism. Endocrine 80, 183–190 (2023). https://doi.org/10.1007/s12020-022-03265-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-022-03265-8