Abstract
Purpose
The early diagnosis of lymph node metastasis (LNM) in papillary thyroid carcinoma (PTC) is clinically important, as it can aid in treatment decision-making and improve prognosis. In the present study, we aimed to identify whether plasma exosomal miRNAs could be potential diagnostic markers of LNM in PTC.
Methods
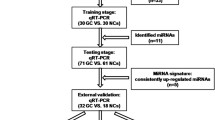
Profiles of plasma exosomal miRNAs were screened using miRNA microarrays. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed in the validation and diagnostic sets to select candidate exosomal miRNAs. Finally, receiver operating characteristic (ROC) curves were generated to evaluate the efficiency of target exosomal miRNAs in distinguishing PTC-N1 patients from PTC-N0 patients.
Results
In total, 197 miRNAs were found to be differentially expressed in the testing set. Based on the qRT-PCR results, the expression of miR-6774-3p (p < 0.001) and miR-6879-5p (p < 0.001) in the PTC-N1 patients was significantly higher than that in the controls. The AUC values of plasma exosomal miR-6774-3p (0.802; 95% CI, 0.724–0.879) and miR-6879-5p (0.787; 95% CI, 0.706–0.867) and their combination (0.914; 95% CI, 0.865–0.962) were higher than those of the total miRNAs directly isolated from plasma. Moreover, the expression of exosomal miRNAs was stable after treatment with RNase A, prolonged incubation, or repeated freezing and thawing.
Conclusions
The two plasma exosomal miRNAs (miR-6774-3p and miR-6879-5p) and their combination could serve as new promising biomarkers for the diagnosis of LNM in PTC patients.





Similar content being viewed by others
Data availability
The datasets generated in this study are available upon request from the corresponding author.
Abbreviations
- PTC:
-
papillary thyroid carcinoma;
- LNM:
-
lymph node metastasis;
- HT:
-
Hashimoto’s thyroiditis;
- miRNAs:
-
microRNAs;
- UTR:
-
untranslated region;
- qRT-PCR:
-
quantitative real-time polymerase chain reaction;
- FNA:
-
fine needle aspiration;
- NTA:
-
nanoparticle tracking analysis;
- TEM:
-
transmission electron microscope;
- AGCC:
-
Affymetrix GeneChip Command Console;
- GO:
-
Gene Ontology;
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes;
- ROC:
-
receiver operating characteristic;
- AUC:
-
area under the curve;
- CI:
-
confidence interval.
References
B.R. Haugen, E.K. Alexander, K.C. Bible, G.M. Doherty, S.J. Mandel, Y.E. Nikiforov et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 26, 1–133 (2016).
R.L. Siegel, K.D. Miller, A. Jemal, Cancer statistics, 2020. CA: A Cancer J. Clin. 70, 7–30 (2020).
T.J. Robinson, S. Thomas, M.A. Dinan, S. Roman, J.A. Sosa, T. Hyslop, How Many Lymph Nodes Are Enough? Assessing the Adequacy of Lymph Node Yield for Papillary Thyroid Cancer. J. Clin. Oncol. 34, 3434–3439 (2016).
Iwadate M., Mitsutake N., Matsuse M., Fukushima T., Suzuki S., Matsumoto Y., et al. The Clinicopathological Results of Thyroid Cancer With BRAFV600E Mutation in the Young Population of Fukushima. J. Clin. Endocrinol. Metab. 105, dgaa573 (2020).
C.W. Lee, J.L. Roh, G. Gong, K.J. Cho, S.H. Choi, S.Y. Nam et al. Risk factors for recurrence of papillary thyroid carcinoma with clinically node-positive lateral neck. Ann. Surg. Oncol. 22, 117–124 (2015).
D.M. Hartl, S. Leboulleux, A. Al Ghuzlan, E. Baudin, L. Chami, M. Schlumberger et al. Optimization of staging of the neck with prophylactic central and lateral neck dissection for papillary thyroid carcinoma. Ann. Surg. 255, 777–783 (2012).
C. Resende de Paiva, C. Gronhoj, U. Feldt-Rasmussen, C. von Buchwald, Association between Hashimoto’s Thyroiditis and Thyroid Cancer in 64,628 Patients. Front. Oncol 7, 53 (2017).
R.M. Tuttle, R.I. Haddad, D.W. Ball, D. Byrd, P. Dickson, Q.Y. Duh et al. Thyroid carcinoma, version 2.2014. J. Natl. Compre. Cancer Network: JNCCN. 12(12), 1671–1680 (2014).
L. Jianyong, Z. Jinjing, L. Zhihui, W. Tao, G. Rixiang, Z. Jingqiang, A Nomogram Based on the Characteristics of Metastatic Lymph Nodes to Predict Papillary Thyroid Carcinoma Recurrence. Thyroid. 28, 301–310 (2018).
A.V. Vlassov, S. Magdaleno, R. Setterquist, R. Conrad, Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochimica et biophysica acta. 1820, 940–948 (2012).
Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 367, aau6977 (2020).
R.C. Friedman, K.K. Farh, C.B. Burge, D.P. Bartel, Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19(1), 92–105 (2009).
D.P. Bartel, MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
J.X. Xie, X. Fan, C.A. Drummond, R. Majumder, Y. Xie, T. Chen et al. MicroRNA profiling in kidney disease: Plasma versus plasma-derived exosomes. Gene 627, 1–8 (2017).
C.F. Zhou, J. Ma, L. Huang, H.Y. Yi, Y.M. Zhang, X.G. Wu et al. Cervical squamous cell carcinoma-secreted exosomal miR-221-3p promotes lymphangiogenesis and lymphatic metastasis by targeting VASH1. Oncogene 38, 1256–1268 (2019).
Y. Zhang, T. Han, D. Feng, J. Li, M. Wu, X. Peng et al. Screening of non-invasive miRNA biomarker candidates for metastasis of gastric cancer by small RNA sequencing of plasma exosomes. Carcinogenesis 41, 582–590 (2020).
T.Y. Huang, C.Y. Wang, K.Y. Chen, L.T. Huang, Urinary Exosomal Thyroglobulin in Thyroid Cancer Patients With Post-ablative Therapy: A New Biomarker in Thyroid Cancer. Front Endocrinol 11, 382 (2020).
M. Liang, S. Yu, S. Tang, L. Bai, J. Cheng, Y. Gu et al. A Panel of Plasma Exosomal miRNAs as Potential Biomarkers for Differential Diagnosis of Thyroid Nodules. Front Genet 11, 449 (2020).
D. Dai, Y. Tan, L. Guo, A. Tang, Y. Zhao, Identification of exosomal miRNA biomarkers for diagnosis of papillary thyroid cancer by small RNA sequencing. Eur J Endocrinol 182, 111–121 (2020).
Q. Pan, J. Zhao, M. Li, X. Liu, Y. Xu, W. Li et al. Exosomal miRNAs are potential diagnostic biomarkers between malignant and benign thyroid nodules based on next-generation sequencing. Carcinogenesis 41, 18–24 (2020).
M. Tang, Q. Wang, K. Wang, F. Wang, Mesenchymal stem cells-originated exosomal microRNA-152 impairs proliferation, invasion and migration of thyroid carcinoma cells by interacting with DPP4. J Endocrinol invest. 43, 1787–1796 (2020).
Capriglione F., Verrienti A., Celano M., Maggisano V., Sponziello M., Pecce V., et al. Analysis of serum microRNA in exosomal vehicles of papillary thyroid cancer. Endocrine. 2021. https://doi.org/10.1007/s12020-021-02847-2.
K. Jiang, G. Li, W. Chen, L. Song, T. Wei, Z. Li et al. Plasma Exosomal miR-146b-5p and miR-222-3p are Potential Biomarkers for Lymph Node Metastasis in Papillary Thyroid Carcinomas. Onco Targets Ther. 13, 1311–1319 (2020).
G. Li, R. Li, L. Song, W. Chen, K. Jiang, H. Tang et al. Implications of Extrathyroidal Extension Invading Only the Strap Muscles in Papillary Thyroid Carcinomas. Thyroid 30(1), 57–64 (2020).
M. Ya, [Interpretation of the management guidelines for patients with thyroid nodules and differentiated thyroid cancer (2012 Chinese edition)]. Lin chuang er bi yan hou tou jing wai ke za zhi = Journal of clinical otorhinolaryngology, head, and neck surgery 27, 917–920 (2013).
L. Min, S. Zhu, L. Chen, X. Liu, R. Wei, L. Zhao et al. Evaluation of circulating small extracellular vesicles derived miRNAs as biomarkers of early colon cancer: a comparison with plasma total miRNAs. J. Extracell. Vesicles 8, 1643670 (2019).
C. Thery, K.W. Witwer, E. Aikawa, M.J. Alcaraz, J.D. Anderson, R. Andriantsitohaina et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7, 1535750 (2018).
H.J. Peltier, G.J. Latham,, Normalization of microRNA expression levels in quantitative RT-PCR assays: identification of suitable reference RNA targets in normal and cancerous human solid tissues. RNA 14, 844–852 (2008).
Y. Li, G.M. Xiang, L.L. Liu, C. Liu, F. Liu, D.N. Jiang et al. Assessment of endogenous reference gene suitability for serum exosomal microRNA expression analysis in liver carcinoma resection studies. Mol. Med. Rep. 12, 4683–4691 (2015).
V. Severino, J.M. Dumonceau, M. Delhaye, S. Moll, I. Annessi-Ramseyer, X. Robin et al. Extracellular Vesicles in Bile as Markers of Malignant Biliary Stenoses. Gastroenterology 153, 495–504 (2017).
L. Zhu, J. Li, Y. Gong, Q. Wu, S. Tan, D. Sun et al. Exosomal tRNA-derived small RNA as a promising biomarker for cancer diagnosis. Mol. Cancer 18, 74 (2019).
H. Fu, H. Yang, X. Zhang, B. Wang, J. Mao, X. Li et al. Exosomal TRIM3 is a novel marker and therapy target for gastric cancer. J. Exp. Cancer Res: CR 37, 162 (2018).
A.O. Batagov, Kurochkin IV. Exosomes secreted by human cells transport largely mRNA fragments that are enriched in the 3’-untranslated regions. Biol Direct 8, 12 (2013).
C. Villarroya-Beltri, C. Gutierrez-Vazquez, F. Sanchez-Cabo, D. Perez-Hernandez, J. Vazquez, N. Martin-Cofreces et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 4, 2980 (2013).
L. Santangelo, G. Giurato, C. Cicchini, C. Montaldo, C. Mancone, R. Tarallo et al. The RNA-Binding Protein SYNCRIP Is a Component of the Hepatocyte Exosomal Machinery Controlling MicroRNA Sorting. Cell Rep. 17, 799–808 (2016).
X. Guo, X. Lv, Y. Ru, F. Zhou, N. Wang, H. Xi et al. Circulating Exosomal Gastric Cancer-Associated Long Noncoding RNA1 as a Biomarker for Early Detection and Monitoring Progression of Gastric Cancer: A Multiphase Study. JAMA Surg. 155, 572–579 (2020).
T. Baranyai, K. Herczeg, Z. Onodi, I. Voszka, K. Modos, N. Marton et al. Isolation of Exosomes from Blood Plasma: Qualitative and Quantitative Comparison of Ultracentrifugation and Size Exclusion Chromatography Methods. PloS ONE 10, e0145686 (2015).
M. Ding, C. Wang, X. Lu, C. Zhang, Z. Zhou, X. Chen et al. Comparison of commercial exosome isolation kits for circulating exosomal microRNA profiling. Anal. Bioanal. Chem. 410, 3805–3814 (2018).
Shirakami Y., Iwashita T., Uemura S., Imai H., Murase K., Shimizu M. Micro-RNA Analysis of Pancreatic Cyst Fluid for Diagnosing Malignant Transformation of Intraductal Papillary Mucinous Neoplasm by Comparing Intraductal Papillary Mucinous Adenoma and Carcinoma. J. Clin. Med. 10, 2249 (2021).
Acknowledgements
We thank the patients for participating in this study. We thank Echo Biotech Co., Ltd, Beijing, China for its technical support in the identification of exosomes (NTA, TEM and western blot analysis).
Author contributors
W.J.C.: study concept and design, experimental studies, data analysis, and drafting of the manuscript; G.P.L.: study concept and design, experimental studies, and statistical analysis; Z.H.L.: administrative or technical support and study supervision; J.Q.Z.: administrative or technical support and study supervision; T.W.: administrative or technical support and study supervision; J.Y.L.: study concept and design, critical revision of the manuscript, and study supervision. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation (82173245), Sichuan Science and Technology Program (2020YFS0208), the 1•3•5 Project for Disciplines of Excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (2021HXFH005), the Science and Technology Achievement Transformation Project, West China Hospital, Sichuan University (CGZH21004), and Sichuan Science and Technology Program (2020YJ0237).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Consent to participate
Written informed consent was obtained from the participants for participating in this study.
Consent for publication
Written informed consent was obtained from the participants for the publication of any potentially identifiable images or data included in this article.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of West China Hospital of Sichuan University (20190214).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Chen, W., Li, G., Li, Z. et al. Evaluation of plasma exosomal miRNAs as potential diagnostic biomarkers of lymph node metastasis in papillary thyroid carcinoma. Endocrine 75, 846–855 (2022). https://doi.org/10.1007/s12020-021-02949-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-021-02949-x