Abstract
Purpose
Both prolactinomas and nonfunctioning adenomas (NFAs) can present with hyperprolactinemia. Distinguishing them is critical because prolactinomas are effectively managed with dopamine agonists, whereas compressive NFAs are treated surgically. Current guidelines rely only on serum prolactin (PRL) levels, which are neither sensitive nor specific enough. Recent studies suggest that accounting for tumor volume may improve diagnosis. The objective of this study is to investigate the diagnostic utility of PRL, tumor volume, and imaging features in differentiating prolactinoma and NFA.
Methods
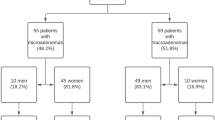
Adult patients with pathologically confirmed prolactinoma (n = 21) or NFA with hyperprolactinemia (n = 58) between 2013 and 2020 were retrospectively identified. Diagnostic performance of clinical and imaging variables was analyzed using receiver-operating characteristic curves to calculate area under the curve (AUC).
Results
Tumor volume and PRL positively correlated for prolactinoma (r = 0.4839, p = 0.0263) but not for NFA (r = 0.0421, p = 0.7536). PRL distinguished prolactinomas from NFAs with an AUC of 0.8892 (p < 0.0001) and optimal cut-off value of 62.45 ng/ml, yielding a sensitivity of 85.71% and specificity of 94.83%. The ratio of PRL to tumor volume had an AUC of 0.9647 (p < 0.0001) and optimal cut-off value of 21.62 (ng/ml)/cm3 with sensitivity of 100% and specificity of 82.76%. Binary logistic regression found that PRL was a significant positive predictor of prolactinoma diagnosis, whereas tumor volume, presence of cavernous sinus invasion, and T2 hyperintensity were significant negative predictors. The regression model had an AUC of 0.9915 (p < 0.0001).
Conclusions
Consideration of tumor volume improves differentiation between prolactinomas and NFAs, which in turn leads to effective management.



Similar content being viewed by others
Change history
05 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12020-021-02811-0
07 June 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12020-021-02777-z
References
A.F. Daly, M. Rixhon, C. Adam, A. Dempegioti, M.A. Tichomirowa, A. Beckers, High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J. Clin. Endocrinol. Metab. 91(12), 4769–4775 (2006)
J.A. Romijn, Hyperprolactinemia and prolactinoma. Handb. Clin. Neurol. 124, 185–195 (2014)
M.E. Molitch, Disorders of prolactin secretion. Endocrinol. Metab. Clin. North Am. 30(3), 585–610 (2001)
A. Glezer, M.D. Bronstein, Prolactinomas. Endocrinol. Metab. Clin. North Am. 44(1), 71–78 (2015)
D.L. Penn, W.T. Burke, E.R. Laws, Management of non-functioning pituitary adenomas: surgery. Pituitary 21(2), 145–153 (2018)
S. Melmed, F.F. Casanueva, A.R. Hoffman et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 96(2), 273–288 (2011)
L. Vilar, C.F. Vilar, R. Lyra, M.D.C. Freitas, Pitfalls in the diagnostic evaluation of hyperprolactinemia. Neuroendocrinology 109(1), 7–19 (2019)
I. Samperi, K. Lithgow, N. Karavitaki, Hyperprolactinaemia. J. Clin. Med 8, 12 (2019)
Y. Huang, C. Ding, F. Zhang, D. Xiao, L. Zhao, S. Wang, Role of prolactin/adenoma maximum diameter and prolactin/adenoma volume in the differential diagnosis of prolactinomas and other types of pituitary adenomas. Oncol. Lett. 15(2), 2010–2016 (2018)
Burke WT, Penn DL, Castlen JP, et al. Prolactinomas and nonfunctioning adenomas: preoperative diagnosis of tumor type using serum prolactin and tumor size. J Neurosurg. 1–8 (2019)
L. Kotsis, Z. Krisár, J. Imre, M. Csikos, Complications of oesophagoplasty with isoperistaltic transverse colon. Scand. J. Thorac. Cardiovasc. Surg. 17(3), 317–321 (1983)
E. Knosp, E. Steiner, K. Kitz, C. Matula, Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33(4), 610–617 (1993). discussion 617–618
A.S. Micko, A. Wohrer, S. Wolfsberger, E. Knosp, Invasion of the cavernous sinus space in pituitary adenomas: endoscopic verification and its correlation with an MRI-based classification. J. Neurosurg. 122(4), 803–811 (2015)
F. Zhang, Y. Huang, C. Ding, G. Huang, S. Wang, The prevalence of hyperprolactinemia in non-functioning pituitary macroadenomas. Int J. Clin. Exp. Med 8(10), 18990–18997 (2015)
M.V. Smith, E.R. Laws Jr, Magnetic resonance imaging measurements of pituitary stalk compression and deviation in patients with nonprolactin-secreting intrasellar and parasellar tumors: lack of correlation with serum prolactin levels. Neurosurgery 34(5), 834–839 (1994). discussion 839
N. Karavitaki, G. Thanabalasingham, H.C. Shore et al. Do the limits of serum prolactin in disconnection hyperprolactinaemia need re-definition? A study of 226 patients with histologically verified non-functioning pituitary macroadenoma. Clin. Endocrinol. 65(4), 524–529 (2006)
T. Kawaguchi, Y. Ogawa, T. Tominaga, Diagnostic pitfalls of hyperprolactinemia: the importance of sequential pituitary imaging. BMC Res Notes 7, 555 (2014)
A. Hagiwara, Y. Inoue, K. Wakasa, T. Haba, T. Tashiro, T. Miyamoto, Comparison of growth hormone-producing and non-growth hormone-producing pituitary adenomas: imaging characteristics and pathologic correlation. Radiology 228(2), 533–538 (2003)
M. Gruppetta, J. Vassallo, Epidemiology and radiological geometric assessment of pituitary macroadenomas: population-based study. Clin. Endocrinol. 85(2), 223–231 (2016)
E.V. Varlamov, J.M. Hinojosa-Amaya, M. Fleseriu, Magnetic resonance imaging in the management of prolactinomas; a review of the evidence. Pituitary 23(1), 16–26 (2020)
J. Kreutz, L. Vroonen, F. Cattin et al. Intensity of prolactinoma on T2-weighted magnetic resonance imaging: towards another gender difference. Neuroradiology 57(7), 679–684 (2015)
S.C. Dogansen, G.Y. Yalin, S. Tanrikulu et al. Clinicopathological significance of baseline T2-weighted signal intensity in functional pituitary adenomas. Pituitary 21(4), 347–354 (2018)
M.C. Burlacu, D. Maiter, T. Duprez, E. Delgrange, T2-weighted magnetic resonance imaging characterization of prolactinomas and association with their response to dopamine agonists. Endocrine 63(2), 323–331 (2019)
M.B.S. Lopes, The 2017 World Health Organization classification of tumors of the pituitary gland: a summary. Acta Neuropathol. 134(4), 521–535 (2017)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kyla Wright, Dr. Matthew Lee, Natalie Escobar, Dr. Donato Pacione, Dr. Girish Fatterpekar, and Dr. Matthew Young have nothing to disclose. Dr. Nidhi Agrawal is on the advisory board for Corcept Therapeutics and is a principal investigator for institution-directed research grants (Chiasm Acromegaly Registry).
Ethical approval
This study was approved by the institutional review board and carried out in accordance with the approved guidelines.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wright, K., Lee, M., Escobar, N. et al. Tumor volume improves preoperative differentiation of prolactinomas and nonfunctioning pituitary adenomas. Endocrine 74, 138–145 (2021). https://doi.org/10.1007/s12020-021-02744-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-021-02744-8