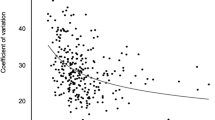
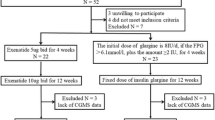
Abstract
Aims
The association between β-cell function and glycemic variability remains to be clarified in insulin-treated patients with type 2 diabetes. Therefore, the study sought to examine the association of various indices of β-cell function with glycemic variability in Chinese insulin-treated patients with type 2 diabetes.
Methods
Glycemic variability was assessed by the coefficient of variation (CV) of glucose levels with the use of continuous glucose monitoring (CGM). Basal β-cell function was evaluated by fasting C-peptide (FCP) and the homeostasis model assessment 2 for β-cell function (HOMA2-%β). Postload β-cell function was measured by 2-hour C-peptide (2hCP) and the acute C-peptide response (ACPR) to arginine.
Results
When a cutoff value of CV ≥ 36% was used to define unstable glucose, the multivariable-adjusted odds ratios for labile glycemic control were 0.34 (95% CI 0.18–0.64) for each 1 ng/mL increase in ACPR, 0.47 (95% CI 0.27–0.81) for each 1 ng/mL increase in FCP, 0.77 (95% CI 0.61–0.97) for each 1 ng/mL increase in 2hCP, and 1.00 (95% CI 0.98–1.01) for each 1% increase in HOMA2-%β. When we further adjusted for 2hCP and HOMA2-%β in the ACPR and FCP analyses, and adjusted for ACPR or FCP in the 2hCP analyses, only ACPR but not FCP or 2hPC remained to be a significant and inverse predictor for labile glycemic control.
Conclusions
ACPR evaluated by the arginine stimulation test may be superior to other commonly used β-cell function parameters to reflect glycemic fluctuation in insulin-treated patients with type 2 diabetes.

Similar content being viewed by others
Abbreviations
- 2hCP:
-
2-hour C-peptide
- 2hPG:
-
2-hour plasma glucose
- ACPR:
-
acute C-peptide response
- AST:
-
arginine stimulation test
- BMI:
-
body mass index
- CGM:
-
continuous glucose monitoring
- CV:
-
coefficient of variation
- FCP:
-
fasting C-peptide
- FPG:
-
fasting plasma glucose
- HbA1c :
-
glycated hemoglobin A1c
- HDL-c:
-
high-density lipoprotein cholesterol
- HOMA2-%β:
-
homeostasis model assessment 2 for β-cell function
- HOMA2-IR:
-
homeostasis model assessment 2 of insulin resistance
- LDL-c:
-
low-density lipoprotein cholesterol
- TC:
-
total cholesterol
- TGs:
-
triglycerides.
References
L. Monnier, E. Mas, C. Ginet, F. Michel, L. Villon, J.P. Cristol, C. Colette, Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 295(14), 1681–1687 (2006). https://doi.org/10.1001/jama.295.14.1681
W. Xu, Y. Zhu, X. Yang, H. Deng, J. Yan, S. Lin, H. Yang, H. Chen, J. Weng, Glycemic variability is an important risk factor for cardiovascular autonomic neuropathy in newly diagnosed type 2 diabetic patients. Int. J. Cardiol. 215, 263–268 (2016). https://doi.org/10.1016/j.ijcard.2016.04.078
J. Lu, X. Ma, L. Zhang, Y. Mo, L. Ying, W. Lu, W. Zhu, Y. Bao, J. Zhou, Glycemic variability assessed by continuous glucose monitoring and the risk of diabetic retinopathy in latent autoimmune diabetes of the adult and type 2 diabetes. J. Diabetes Investig. 10(3), 753–759 (2019). https://doi.org/10.1111/jdi.12957
A. Akirov, T. Diker-Cohen, H. Masri-Iraqi, I. Shimon, High glucose variability increases mortality risk in hospitalized patients. J. Clin. Endocrinol. Metab. 102(7), 2230–2241 (2017). https://doi.org/10.1210/jc.2017-00450
J. Shen, Z. Chen, C. Chen, X. Zhu, Y. Han, Impact of incretin on early-phase insulin secretion and glucose excursion. Endocrine 44(2), 403–410 (2013). https://doi.org/10.1007/s12020-012-9867-9
J. Peng, J. Lu, X. Ma, L. Ying, W. Lu, W. Zhu, Y. Bao, J. Zhou, Breakfast replacement with a liquid formula improves glycemic variability in patients with type 2 diabetes: a randomized clinical trial. Br. J. Nutr. 1–25 (2018). https://doi.org/10.1017/S0007114518003628
M. Kanat, A. Mari, L. Norton, D. Winnier, R.A. DeFronzo, C. Jenkinson, M.A. Abdul-Ghani, Distinct beta-cell defects in impaired fasting glucose and impaired glucose tolerance. Diabetes 61(2), 447–453 (2012). https://doi.org/10.2337/db11-0995
K.D. Kohnert, P. Augstein, E. Zander, P. Heinke, K. Peterson, E.J. Freyse, R. Hovorka, E. Salzsieder, Glycemic variability correlates strongly with postprandial beta-cell dysfunction in a segment of type 2 diabetic patients using oral hypoglycemic agents. Diabetes Care 32(6), 1058–1062 (2009). https://doi.org/10.2337/dc08-1956
C.K. Kramer, H. Choi, B. Zinman, R. Retnakaran, Glycemic variability in patients with early type 2 diabetes: the impact of improvement in beta-cell function. Diabetes Care 37(4), 1116–1123 (2014). https://doi.org/10.2337/dc13-2591
K.D. Kohnert, P. Heinke, L. Vogt, P. Augstein, E. Salzsieder, Declining ss-cell function is associated with the lack of long-range negative correlation in glucose dynamics and increased glycemic variability: a retrospective analysis in patients with type 2 diabetes. J. Clin. Transl. Endocrinol. 1(4), 192–199 (2014). https://doi.org/10.1016/j.jcte.2014.09.003
T.P. Solomon, S.K. Malin, K. Karstoft, S.R. Kashyap, J.M. Haus, J.P. Kirwan, Pancreatic beta-cell function is a stronger predictor of changes in glycemic control after an aerobic exercise intervention than insulin sensitivity. J. Clin. Endocrinol. Metab. 98(10), 4176–4186 (2013). https://doi.org/10.1210/jc.2013-2232
F.S. Fang, X.L. Cheng, Y.P. Gong, L.C. Wang, L. Li, J. Li, H. Tian, C.L. Li, Association between glycemic indices and beta cell function in patients with newly diagnosed type 2 diabetes. Curr. Med. Res. Opin. 30(8), 1437–1440 (2014). https://doi.org/10.1185/03007995.2014.918030
T. Chen, F. Xu, J.B. Su, X.Q. Wang, J.F. Chen, G. Wu, Y. Jin, X.H. Wang, Glycemic variability in relation to oral disposition index in the subjects with different stages of glucose tolerance. Diabetol. Metab. Syndr. 5, 38 (2013). https://doi.org/10.1186/1758-5996-5-38
S.S. Shankar, A. Vella, R.H. Raymond, M.A. Staten, R.A. Calle, R.N. Bergman, C. Cao, D. Chen, C. Cobelli, C. Dalla Man, M. Deeg, J.Q. Dong, D.S. Lee, D. Polidori, R.P. Robertson, H. Ruetten, D. Stefanovski, M.T. Vassileva, G.C. Weir, D.A. Fryburg, Standardized mixed-meal tolerance and arginine stimulation tests provide reproducible and complementary measures of beta-cell function: results from the Foundation for the National Institutes of Health Biomarkers Consortium Investigative Series. Diabetes Care 39(9), 1602–1613 (2016). https://doi.org/10.2337/dc15-0931
H. Larsson, G. Berglund, B. Ahren, Glucose modulation of insulin and glucagon secretion is altered in impaired glucose tolerance. J. Clin. Endocrinol. Metab. 80(6), 1778–1782 (1995). https://doi.org/10.1210/jcem.80.6.7775622
H. Larsson, B. Ahren, Glucose-dependent arginine stimulation test for characterization of islet function: studies on reproducibility and priming effect of arginine. Diabetologia 41(7), 772–777 (1998). https://doi.org/10.1007/s001250050986
T. Danne, R. Nimri, T. Battelino, R.M. Bergenstal, K.L. Close, J.H. DeVries, S. Garg, L. Heinemann, I. Hirsch, S.A. Amiel, R. Beck, E. Bosi, B. Buckingham, C. Cobelli, E. Dassau, F.J. Doyle 3rd, S. Heller, R. Hovorka, W. Jia, T. Jones, O. Kordonouri, B. Kovatchev, A. Kowalski, L. Laffel, D. Maahs, H.R. Murphy, K. Norgaard, C.G. Parkin, E. Renard, B. Saboo, M. Scharf, W.V. Tamborlane, S.A. Weinzimer, M. Phillip, International consensus on use of continuous glucose monitoring. Diabetes Care 40(12), 1631–1640 (2017). https://doi.org/10.2337/dc17-1600
J.R. Petrie, A.L. Peters, R.M. Bergenstal, R.W. Holl, G.A. Fleming, L. Heinemann, Improving the clinical value and utility of CGM systems: issues and recommendations: a joint statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetologia 60(12), 2319–2328 (2017). https://doi.org/10.1007/s00125-017-4463-4
T. Battelino, T. Danne, R.M. Bergenstal, S.A. Amiel, R. Beck, T. Biester, E. Bosi, B.A. Buckingham, W.T. Cefalu, K.L. Close, C. Cobelli, E. Dassau, J.H. DeVries, K.C. Donaghue, K. Dovc, F.J. Doyle 3rd, S. Garg, G. Grunberger, S. Heller, L. Heinemann, I.B. Hirsch, R. Hovorka, W. Jia, O. Kordonouri, B. Kovatchev, A. Kowalski, L. Laffel, B. Levine, A. Mayorov, C. Mathieu, H.R. Murphy, R. Nimri, K. Norgaard, C.G. Parkin, E. Renard, D. Rodbard, B. Saboo, D. Schatz, K. Stoner, T. Urakami, S.A. Weinzimer, M. Phillip, Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 42(8), 1593–1603 (2019). https://doi.org/10.2337/dci19-0028
American Diabetes Association. Standards of medical care in diabetes–2010. Diabetes Care 33(Suppl 1), S11–S61 (2010). https://doi.org/10.2337/dc10-S011
G. Boden, J. Ruiz, C.J. Kim, X. Chen, Effects of prolonged glucose infusion on insulin secretion, clearance, and action in normal subjects. Am. J. Physiol. 270(2 Pt 1), E251–E258 (1996). https://doi.org/10.1152/ajpendo.1996.270.2.E251
C. Hu, C. Wang, R. Zhang, X. Ma, J. Wang, J. Lu, W. Qin, Y. Bao, K. Xiang, W. Jia, Variations in KCNQ1 are associated with type 2 diabetes and beta cell function in a Chinese population. Diabetologia 52(7), 1322–1325 (2009). https://doi.org/10.1007/s00125-009-1335-6
J. Lu, X. Ma, J. Zhou, L. Zhang, Y. Mo, L. Ying, W. Lu, W. Zhu, Y. Bao, R.A. Vigersky, W. Jia, Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 41(11), 2370–2376 (2018). https://doi.org/10.2337/dc18-1131
J.C. Levy, D.R. Matthews, M.P. Hermans, Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 21(12), 2191–2192 (1998). https://doi.org/10.2337/diacare.21.12.2191
E. Gerbaud, R. Darier, M. Montaudon, M.C. Beauvieux, C. Coffin-Boutreux, P. Coste, H. Douard, A. Ouattara, B. Catargi, Glycemic variability is a powerful independent predictive factor of midterm major adverse cardiac events in patients with diabetes with acute coronary syndrome. Diabetes Care 42(4), 674–681 (2019). https://doi.org/10.2337/dc18-2047
H. Takahashi, N. Iwahashi, J. Kirigaya, S. Kataoka, Y. Minamimoto, M. Gohbara, T. Abe, K. Okada, Y. Matsuzawa, M. Konishi, N. Maejima, K. Hibi, M. Kosuge, T. Ebina, K. Tamura, K. Kimura, Glycemic variability determined with a continuous glucose monitoring system can predict prognosis after acute coronary syndrome. Cardiovasc Diabetol. 17(1), 116 (2018). https://doi.org/10.1186/s12933-018-0761-5
S. Frontoni, P. Di Bartolo, A. Avogaro, E. Bosi, G. Paolisso, A. Ceriello, Glucose variability: an emerging target for the treatment of diabetes mellitus. Diabetes Res. Clin. Pract. 102(2), 86–95 (2013). https://doi.org/10.1016/j.diabres.2013.09.007
S.O. Oyibo, Y.D. Prasad, N.J. Jackson, E.B. Jude, A.J. Boulton, The relationship between blood glucose excursions and painful diabetic peripheral neuropathy: a pilot study. Diabet. Med. 19(10), 870–873 (2002)
J. Smith-Palmer, M. Brandle, R. Trevisan, M. Orsini Federici, S. Liabat, W. Valentine, Assessment of the association between glycemic variability and diabetes-related complications in type 1 and type 2 diabetes. Diabetes Res. Clin. Pract. 105(3), 273–284 (2014). https://doi.org/10.1016/j.diabres.2014.06.007
X. Tang, S. Li, Y. Wang, M. Wang, Q. Yin, P. Mu, S. Lin, X. Qian, X. Ye, Y. Chen, Glycemic variability evaluated by continuous glucose monitoring system is associated with the 10-y cardiovascular risk of diabetic patients with well-controlled HbA1c. Clin. Chim. Acta 461, 146–150 (2016). https://doi.org/10.1016/j.cca.2016.08.004
G. Yuan, H. Hu, S. Wang, Q. Yang, S. Yu, W. Sun, W. Qian, C. Mao, L. Zhou, D. Chen, Z. Wang, Q. Gong, D. Wang, Improvement of beta-cell function ameliorated glycemic variability in patients with newly diagnosed type 2 diabetes after short-term continuous subcutaneous insulin infusion or in combination with sitagliptin treatment: a randomized control trial. Endocr. J. 62(9), 817–834 (2015). https://doi.org/10.1507/endocrj.EJ15-0160
A.M. Marker, A.E. Noser, M.A. Clements, S.R. Patton, Shared responsibility for type 1 diabetes care is associated with glycemic variability and risk of glycemic excursions in youth. J. Pediatr. Psychol. 43(1), 61–71 (2018). https://doi.org/10.1093/jpepsy/jsx081
Y. Huang, C. Heng, J. Wei, X. Jing, X. Wang, G. Zhao, J. Hou, Q. Liu, K. Jiao, Influencing factors of glycemic variability in hospitalized type 2 diabetes patients with insulin therapy: a Strobe-compliant article. Medicine 96(36), e8021 (2017). https://doi.org/10.1097/MD.0000000000008021
M.B. Christensen, P. Gaede, E. Hommel, A. Gotfredsen, K. Norgaard, Glycaemic variability and hypoglycaemia are associated with C-peptide levels in insulin-treated type 2 diabetes. Diabetes Metab. (2019). https://doi.org/10.1016/j.diabet.2019.02.002
B.L. Wajchenberg, Beta-cell failure in diabetes and preservation by clinical treatment. Endocr. Rev. 28(2), 187–218 (2007). https://doi.org/10.1210/10.1210/er.2006-0038
C. Greenbaum, K. Seidel, C. Pihoker, The case for intravenous arginine stimulation in lieu of mixed-meal tolerance tests as outcome measure for intervention studies in recent-onset type 1 diabetes. Diabetes Care 27(5), 1202–1204 (2004). https://doi.org/10.2337/diacare.27.5.1202
L.B. Harrison, B. Adams-Huet, P. Raskin, I. Lingvay, Beta-cell function preservation after 3.5 years of intensive diabetes therapy. Diabetes Care 35(7), 1406–1412 (2012). https://doi.org/10.2337/dc11-2170
J. Weng, Y. Li, W. Xu, L. Shi, Q. Zhang, D. Zhu, Y. Hu, Z. Zhou, X. Yan, H. Tian, X. Ran, Z. Luo, J. Xian, L. Yan, F. Li, L. Zeng, Y. Chen, L. Yang, S. Yan, J. Liu, M. Li, Z. Fu, H. Cheng, Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 371(9626), 1753–1760 (2008). https://doi.org/10.1016/S0140-6736(08)60762-X
F. Pistrosch, C. Kohler, F. Schaper, W. Landgraf, T. Forst, M. Hanefeld, Effects of insulin glargine versus metformin on glycemic variability, microvascular and beta-cell function in early type 2 diabetes. Acta Diabetol. 50(4), 587–595 (2013). https://doi.org/10.1007/s00592-012-0451-9.
The ORIGIN trial investigators., Characteristics associated with maintenance of mean A1C<6.5% in people with dysglycemia in the ORIGIN trial. Diabetes Care 36(10), 2915–2922 (2013). https://doi.org/10.2337/dc12-2238
L. Ji, P. Zhang, D. Zhu, X. Li, J. Ji, J. Lu, X. Guo, W. Jia, J. Weng, Y. Wu, W. Yang, D. Zou, Z. Zhou, C. Pan, Y. Gao, S.K. Garg, Observational Registry of Basal Insulin Treatment (ORBIT) in patients with type 2 diabetes uncontrolled with oral antihyperglycaemic drugs: real-life use of basal insulin in China. Diabetes Obes. Metab. 19(6), 822–830 (2017). https://doi.org/10.1111/dom.12886
Acknowledgements
We would like to thank all the involved clinicians, nurses, and technicians for helping with the study. We are grateful to all participants for their dedication in data collection and laboratory measurements.
Authors contributions
J.Z. and G.H. conceived and designed the study. Y.S. (Y Si), Y.S. (Y Shen) and J.L. contributed to data collection, data analysis, and writing the paper. Y.S. (Y Si), L.Z. and Y.M. contributed to data analysis. W.L. and W.Z. contributed to conduction of study and data collection. X.M., Y.B. and J.Z. contributed to interpretation of data and revision of the paper. G.H. critically reviewed and edited the paper. All authors revised the paper for important intellectual content and have approved the final version.
Funding
This work was funded by the National Key R&D Program of China (2018YFC2001004), the Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20161430) and Shanghai Municipal Key Clinical Specialty.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants or their legal guardians included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Si, Y., Shen, Y., Lu, J. et al. Impact of acute-phase insulin secretion on glycemic variability in insulin-treated patients with type 2 diabetes. Endocrine 68, 116–123 (2020). https://doi.org/10.1007/s12020-020-02201-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02201-y