Abstract
Purpose
Several new gene mutations have been reported in recent years to be associated with a risk of familial pheochromocytoma. However, it is unclear as to whether extensive genetic testing is required in all patients.
Methods
The clinical data of consecutive patients operated for pheochromocytoma over a decade in a tertiary referral center were reviewed. Genetic screening was performed using a 10-gene panel: RET, VHL, SDHB, SDHD, SDHA, SDHC, SDHAF2, MAX, TMEM127 and FH.
Results
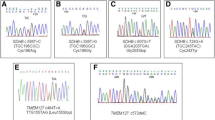
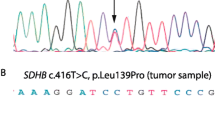
A total of 166 patients were analyzed: 87 of them had genetic screening performed (39 M: 44.8%, 48 F: 55.2%, age range 6–81 years, mean 45±16.8 years). In total, 22/87 (25.3%) patients had germline mutations, while 65/87 (74.7%) patients presented with apparently sporadic tumors. Germline VHL mutations were identified in 11.7% of patients, RET in 6.8% (five MEN2A/MEN2 and one MEN2B/MEN3), SDHD in 2.3%, MAX in 2.3%, SDHB in 1.1%, and TMEM127 in 1.1% of patients. At diagnosis, 15.1% of patients with unilateral non-syndromic pheochromocytoma showed germline mutations. We identified 19.7% of mutations in patients with unilateral-non-recurrent pheochromocytomas within 5 years vs. 50% in the recurrent-bilateral-metastatic group (p = 0.01). Germline mutations were more frequently seen with bilateral pheochromocytomas (p = 0.001): 80% of patients with bilateral disease had germline mutations (4 VHL, 3 RET, 1 MAX).
Conclusions
The advent of rapid genetic screening using a gene-panel makes it feasible to screen large cohorts of patients and provides a valuable tool to contribute to the prediction of bilateral and malignant disease and to screen family members.


Similar content being viewed by others
Abbreviations
- PHEO:
-
Pheochromocytomas
- PGL:
-
Paraganglioma
- NET:
-
Neuroendocrine tumor
- NMA:
-
Normetanephrines
- MA:
-
Metanephrines
- 3MT:
-
3-methoxythyramine
- NGS:
-
Next-generationsequencing
References
J.T. Adler et al., Pheochromocytoma: current approaches and future directions. Oncologist 13(7), 779–793 (2008)
I. Ilias, K. Pacak, A clinical overview of pheochromocytomas/paragangliomas and carcinoid tumors. Nucl. Med. Biol. 35(Suppl 1), S27–S34 (2008)
G. Eisenhofer et al., Pheochromocytoma catecholamine phenotypes and prediction of tumor size and location by use of plasma free metanephrines. Clin. Chem. 51(4), 735–744 (2005)
M.M. Walther, H.R. Keiser, W.M. Linehan, Pheochromocytoma: evaluation, diagnosis, and treatment. World J. Urol. 17(1), 35–39 (1999)
L. Fishbein, K.L. Nathanson, Pheochromocytoma and paraganglioma: understanding the complexities of the genetic background. Cancer Genet. 205(1-2), 1–11 (2012)
V.L. Martucci, K. Pacak, Pheochromocytoma and paraganglioma: diagnosis, genetics, management, and treatment. Curr. Probl. Cancer 38(1), 7–41 (2014)
L. Amar et al., Genetic testing in pheochromocytoma or functional paraganglioma. J. Clin. Oncol. 23(34), 8812–8818 (2005)
N. Burnichon et al., The succinate dehydrogenase genetic testing in a large prospective series of patients with paragangliomas. J. Clin. Endocrinol. Metab. 94(8), 2817–2827 (2009)
K. Pacak, S.J. Wimalawansa, Pheochromocytoma and paraganglioma. Endocr. Pract. 21(4), 406–412 (2015)
H.P. Neumann et al., Germ-line mutations in nonsyndromic pheochromocytoma. N. Engl. J. Med. 346(19), 1459–1466 (2002)
C.H. Lee et al., Genetics of apparently sporadic pheochromocytoma and paraganglioma in a Chinese population. Horm. Metab. Res. 47(11), 833–838 (2015)
D. Viskochil et al., Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell 62(1), 187–192 (1990)
L.M. Mulligan et al., Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 363(6428), 458–460 (1993)
F. Latif et al., Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 260(5112), 1317–1320 (1993)
R.E. Ferrell et al., Hereditary lymphedema: evidence for linkage and genetic heterogeneity. Hum. Mol. Genet. 7(13), 2073–2078 (1998)
S. Niemann, U. Muller, Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat. Genet. 26(3), 268–270 (2000)
D. Astuti et al., Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am. J. Hum. Genet. 69(1), 49–54 (2001)
S. Lee et al., Neuronal apoptosis linked to EglN3 prolyl hydroxylase and familial pheochromocytoma genes: developmental culling and cancer. Cancer Cell. 8(2), 155–167 (2005)
S. Schlisio et al., The kinesin KIF1Bbeta acts downstream from EglN3 to induce apoptosis and is a potential 1p36 tumor suppressor. Genes Dev. 22(7), 884–893 (2008)
H.X. Hao et al., SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science 325(5944), 1139–1142 (2009)
J. Gaal et al., Isocitrate dehydrogenase mutations are rare in pheochromocytomas and paragangliomas. J. Clin. Endocrinol. Metab. 95(3), 1274–1278 (2010)
Y. Qin et al., Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nat. Genet. 42(3), 229–233 (2010)
N. Burnichon et al., SDHA is a tumor suppressor gene causing paraganglioma. Hum. Mol. Genet. 19(15), 3011–3020 (2010)
I. Comino-Mendez et al., Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nat. Genet. 43(7), 663–667 (2011)
C. Yang et al., Somatic mosaicism of EPAS1 mutations in the syndrome of paraganglioma and somatostatinoma associated with polycythemia. Hum. Genome. Var. 2, 15053 (2015)
A. Buffet et al., A decade (2001-2010) of genetic testing for pheochromocytoma and paraganglioma. Horm. Metab. Res. 44(5), 359–366 (2012)
F.M. Brouwers et al., High frequency of SDHB germline mutations in patients with malignant catecholamine-producing paragangliomas: implications for genetic testing. J. Clin. Endocrinol. Metab. 91(11), 4505–4509 (2006)
D.E. Benn et al., Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. J. Clin. Endocrinol. Metab. 91(3), 827–836 (2006)
J.W. Lenders et al., Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 99(6), 1915–1942 (2014)
P.F. Plouin et al., European Society of Endocrinology Clinical Practice Guideline for long-term follow-up of patients operated on for a phaeochromocytoma or a paraganglioma. Eur. J. Endocrinol. 174(5), G1–G10 (2016)
M.E. Robson et al., American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J. Clin. Oncol. 28(5), 893–901 (2010)
R. Martins, M.J. Bugalho, Paragangliomas/Pheochromocytomas: clinically oriented genetic testing. Int. J. Endocrinol. 2014, 794187 (2014)
L. Fishbein et al., Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing. Ann. Surg. Oncol. 20(5), 1444–1450 (2013)
J.P. Brito et al., Testing for germline mutations in sporadic pheochromocytoma/paraganglioma: a systematic review. Clin. Endocrinol. 82(3), 338–345 (2015)
E. Rattenberry et al., A comprehensive next generation sequencing-based genetic testing strategy to improve diagnosis of inherited pheochromocytoma and paraganglioma. J. Clin. Endocrinol. Metab. 98(7), E1248–E1256 (2013)
NGS in PPGL Study Group, R.A. Toledo, N. Burnichon, A. Cascon, D.E. Benn, J.P. Bayley, J. Welander, C.M. Tops, H. Firth, T. Dwight, T. Ercolino, M. Mannelli, G. Opocher, R. Clifton-Bligh, O. Gimm, E.R. Maher, M. Robledo, A.P. Gimenez-Roqueplo, P.L. Dahia, Consensus statement on next-generation-sequencing-based diagnostic testing of hereditary phaeochromocytomas and paragangliomas. Nat. Rev. Endocrinol. 13(4), 233–247. doi:10.1038/nrendo.2016.185
M. Curras-Freixes et al., Recommendations for somatic and germline genetic testing of single pheochromocytoma and paraganglioma based on findings from a series of 329 patients. J. Med. Genet. 52(10), 647–656 (2015)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration, and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Sbardella, E., Cranston, T., Isidori, A.M. et al. Routine genetic screening with a multi-gene panel in patients with pheochromocytomas. Endocrine 59, 175–182 (2018). https://doi.org/10.1007/s12020-017-1310-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-017-1310-9