Abstract
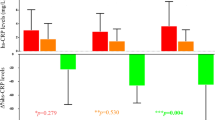
A VLCK diet supplemented with DHA, commercially available, was tested against an isocaloric VLCK diet without DHA. The main purpose of this study was to compare the effect of DHA supplementation in classic cardiovascular risk factors, adipokine levels, and inflammation-resolving eicosanoids. A total of obese patients were randomized into two groups: a group supplemented with DHA (n = 14) (PnK-DHA group) versus a group with an isocaloric diet free of supplementation (n = 15) (control group). The follow-up period was 6 months. The average weight loss after 6 months of treatment was 20.36 ± 5.02 kg in control group and 19.74 ± 5.10 kg in PnK-DHA group, without statistical differences between both groups. The VLCK diets induced a significant change in some of the biological parameters, such as insulin, HOMA-IR, triglycerides, LDL cholesterol, C-reactive protein, resistin, TNF alpha, and leptin. Following DHA supplementation, the DHA-derived oxylipins were significantly increased in the intervention group. The ratio of proresolution/proinflammatory lipid markers was increased in plasma of the intervention group over the entire study. Similarly, the mean ratios of AA/EPA and AA/DHA in erythrocyte membranes were dramatically reduced in the PnK-DHA group and the anti-inflammatory fatty acid index (AIFAI) was consistently increased after the DHA treatment (p < 0.05). The present study demonstrated that a very low-calorie ketogenic diet supplemented with DHA was significantly superior in the anti-inflammatory effect, without statistical differences in weight loss and metabolic improvement.

Similar content being viewed by others
References
K.M. Flegal, M.D. Carroll, B.K. Kit, C.L. Ogden, Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 307, 491–497 (2012)
L. Fontana, S. Klein, Aging, adiposity, and calorie restriction. JAMA 297, 986–994 (2007)
F.B. Hu, J.B. Meigs, T.Y. Li, Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes 53, 693–700 (2004)
E.W. Gregg, H. Chen, L.E. Wagenknecht, J.M. Clark, L.M. Delahanty, Bantle et al., JLook AHEAD Research Group. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 308, 2489–2496 (2012)
L. Sjöström, M. Peltonen, P. Jacobson, C.D. Sjöström, K. Karason, H. Wedel et al., Bariatric surgery and long-term cardiovascular events. JAMA 307, 56–65 (2012)
G.B. Dodell, J.B. Albu, L. Attia, J. McGinty, F.X. Pi-Sunyer, B. Laferrère, The bariatric surgery patient: lost to follow-up; from morbid obesity to severe malnutrition. Endocr. Pract. 18, 21–25 (2012)
W.P. James, I.D. Caterson, W. Coutinho, N. Finer, Gaal L.F. Van, SCOUT Investigators. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N. Engl. J. Med. 363, 905–917 (2010)
J.C. Halford, E.J. Boyland, J.E. Blundell, T.C. Kirkham, J.A. Harrold, Pharmacological management of appetite expression in obesity. Nat. Rev. Endocrinol. 6, 255–269 (2010)
J.A. Harrold, T.M. Dovey, J.E. Blundell, J.C. Halford, CNS regulation of appetite. Neuropharmacology 63, 3–17 (2012)
T.P. Wycherley, L.J. Moran, P.M. Clifton, M. Noakes, G.D. Brinkworthand, Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 96, 1281–1298 (2012)
T.A. Hussain, T.C. Mathew, A.A. Dashti, S. Asfar, N. Al-Zaid, H.M. Dashti, Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition 28, 1016–1021 (2012)
B. Moreno, D. Bellido, I. Sajoux, A. Goday, D. Saavedra, A. Crujeiras, F. Casanueva, Comparison of a very low-calorie-ketogenic diet with a standard low-calorie diet in the treatment of obesity. Endocrine 47(3), 793–805 (2014)
Bakker GC, van Erk MJ, pellins L, Wopereis S, Rubing CM. AN anti-inflammatory dietary mix modulates inflammation and oxidative and metabolic stress In overweight men: a nutrigenomic approach. Am J CLin Nutr 2010:91:1044-1059
R.F. Grimble, Dietary lipids and the inflammatory response. Proc. Nutr. Soc. 57, 535–537 (1998)
C.N. Serhan, N. Chiang, endogenous pro-resolving and anti-inflammatory lipid mediators; a new pharmacological genus. Br. J. Pharmacol. 153(suppl 1), S200–S215 (2008)
SCOOP-VLCD task 7.3. Reports on tasks for scientific cooperation. Collection of data on products intended for use in very-low-calorie-diets. Report. Brussels. European Commission, September 2002
P. Le Faouder, V. Baillif, I. Spreadbury, J.P. Motta, P. Rousset, G. Chêne et al., LC-MS/MS method for rapid and concomitant quantification of pro-inflammatory and pro-resolving polyunsaturated fatty acid metabolites. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 932, 123–133 (2013)
M.J. Duart, C.O. Arroyo, J.L. Moreno, Validation of a insulin model for the reactions in RIA. Clin. Chem. Lab. Med. 40, 1161–1167 (2002)
D.R. Mathews, J.P. Hosker, A.S. Rudenski et al., Homeostasis model assessment: insulin resistance and beta cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–414 (1985)
A. Pfutzner, M. Langefeld, T. Kunt et al., Evaluation of human resistin assays with serum from patients with type 2 diabetes and different degrees of insulin resistance. Clin. Lab. 49, 571–576 (2003)
U. Meier, M. Gressner, Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, Ghrelin, adiponectin, and resistin. Clin. Chem. 50, 1511–1525 (2004)
P. Suominen, Evaluation of an enzyme immunometric assay to measure serum adiponectin concentrations. Clin. Chem. 50, 219–221 (2004)
G. Lepage, C.C. Roy, Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 27, 114–120 (1986)
W.S. Harris, The omega-3 index as a risk factor for coronary heart disease. Am. J. Clin. Nutr. 87(6), 1997S–2002S (2008)
T. Grimstad, R.K. Berge, P. Bohov, J. Skorve, L. Goransson, R. Omdal et al., Salmon diet in patients with active ulcerative colitis reduced the simple clinical colitis activity index and increased the anti-inflammatory fatty acid index—a pilot study. Scand. J. Clin. Lab. Investig. 71(68–73), 32 (2011)
B.T. Kalish, H.D. Le, J.M. Fitzgerald, S. Wang, K. Seamon, K.M. Gura et al., Intravenous fish oil lipid emulsion promotes a shift toward anti-inflammatory proresolving lipid mediators. Am. J. Physiol. Gastrointest. Liver Physiol. 305(11), G818–G828 (2013)
T.A. Wadden, R.H. Neiberg, R.R. Wing, J.M. Clark, L.M. Delahanty, J.O. Hill et al., Four-year weight losses in the look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 19, 1987–1998 (2011)
L. Sjöström, A. Rissanen, T. Andersen, M. Boldrin, A. Golay, H.P. Koppeschaar, M. Krempf, Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. Eur. Multicen. Orlistat Study Group Lancet 352, 167–172 (1998)
J.P. Despres, A. Golay, L. Sjostrom, Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N. Engl. J. Med. 353, 2121–2134 (2005)
L.F. Van Gaal, A.M. Rissanen, A.J. Scheen, O. Ziegler, S. Rossner, For the RIO-Europe Study Group. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1 year experience from the RIO-Europe study. Lancet 365, 1389–1397 (2005)
S. Smith, N.J. Weissman, C.M. Anderson, M. Sanchez, E. Chuang, S. Subbe et al., Multicenter, placebo-controlled trial of lorcaserin for weight management. N. Engl. J. Med. 363, 245–256 (2010)
M. Glandt, I. Raz, Present and future: pharmacologic treatment of Obesity. J. Obes. (2011). doi:10.1155/2011/636181
G.A. Bray, D.H. Ryan, D. Gordon, S. Heidingsfelder, F. Cerise, K. Wilson, A double-blind randomized placebo-controlled trial of sibutramine. Obes. Res. 4, 263–270 (1996)
T. Okasaki, E. Himeno, H. Nanri, H. Ogata, M. Ikeda, Efects of mild aerobic exercise and a mild hypocaloric diet on plasma leptin in sedentary women. Clin. exp. Pharmacol. 26, 415–420 (1999)
J.P. Bastard, C. Jardel, E. Bruckert, Elevated levels of interleukin-6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J. Clin. Endocrinol. Metab. 85, 3338–3342 (2000)
K. Hotta, T. Funahashi, Y. Arita, Plasma concentrations of a novel, adipose specific protein, adiponectin in tyoe 2 patients. Arterioscler. Thromb. Vasc. 20, 1595 (2000)
C. Xenachis, E. Samojlik, M.P. Raghuwanshi, M.A. Kirschner, Leptin, insulin and TNF-alpha in weight loss. J. Endocrinol. Investig. 24, 865–870 (2001)
L.U. Monzillo, O. Hamdy, E.S. Horton, S. Ledbury, C. Mulloly, C. Jarema, S. Porter, K. Ovalle, Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes. Res. 11, 1048–1052 (2003)
G. Schmitz, J. Ecker, The opposing effects of n − 3 and n − 6 fatty acids. Prog. Lipid Res. 47(2), 147–155 (2008)
J. Faber, M. Berkhout, A.P. Vos, J.W. Sijben, P.C. Calder, J. Garssen et al., Supplementation with a fish oil-enriched, high-protein medical food leads to rapid incorporation of EPA into white blood cells and modulates immune responses within one week in healthy men and women. J. Nutr. 141(5), 964–970 (2011)
M.J. James, R.A. Gibson, L.G. Cleland, Dietary polyunsaturated fatty acids and inflammatory mediator production. Am. J. Clin. Nutr. 71(1 Suppl), 343S–348S (2000)
M. Spite, J. Clària, C.N. Serhan, Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 19(1), 21–36 (2014)
C.N. Serhan, Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 25, 101–137 (2007)
M.J. Zhang, M. Spite, Resolvins: anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annu. Rev. Nutr. 32, 203–227 (2012)
P. Flachs, R. Rühl, M. Hensler, P. Janovska, P. Zouhar, V. Kus et al., Synergistic induction of lipid catabolism and anti-inflammatory lipids in white fat of dietary obese mice in response to calorie restriction and n-3 fatty acids. Diabetologia 54(10), 2626–2638 (2011)
Acknowledgments
We acknowledge PNKDIET, SLU, Spain, for providing free of charge the diet of the ketogenic phases in both groups and oral supplementation of DHA or placebo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Glossary
- 12-HETE
-
15-Hydroxyicosatetraenoic acid
- 12-LOX
-
12-Lipoxygenase
- 14-HDOHE
-
14-Hydroxy docosahexaenoic acid
- 14-HDHA
-
14-Hydroxy docosahexaenoic acid
- 15-HETE
-
15-Hydroxyicosatetraenoic acid
- 15-LOX
-
12-Lipoxygenase
- 17-HDOHE
-
17-Hydroxy docosahexaenoic acid
- 17-HDHA
-
17-Hydroxy docosahexaenoic acid
- 4-HDOHE
-
4-Hydroxy docosahexaenoic acid
- 4-HDHA
-
4-Hydroxy docosahexaenoic acid
- 5-HETE
-
5-Hydroxyicosatetraenoic acid
- 5-LOX
-
5-Lipoxygenase
- 7-HDOHE
-
7-Hydroxy docosahexaenoic acid
- 7-HDHA
-
7-Hydroxy docosahexaenoic acid
- 7RMAR1
-
Nuclear matrix-associated protein RMAR1
- 7SMAR1
-
Nuclear matrix-associated protein SMAR1
- 8-HETE
-
8-Hydroxyicosatetraenoic acid
- AA
-
Arachidonic acid
- ADO
-
Available data only
- AIFAI
-
Anti-inflammatory fatty acid index
- ANCOVA
-
Analysis of covariance
- BHT
-
Butylhydroxytoluene
- BMI
-
Body mass index
- CHOL
-
Cholesterol
- COX
-
Cyclooxygenase
- CRP
-
C-reactive protein
- DHA
-
Docosahexaenoic acid
- DPA
-
Docosapentaenoic acid
- EPA
-
Eicosapentaenoic acid
- FAME
-
Fatty acid methyl ester
- GCMS
-
Gas chromatograph/mass spectrometer
- HDL
-
High-density lipoprotein
- HOMA-IR
-
Homeostatic model assessment of insulin resistance
- HPLC
-
High-performance liquid chromatography
- IL6
-
Interleukin 6
- K2-EDTA
-
K2-Ethylenediaminetetraacetic acid
- LC
-
Liquid chromatography
- LDL
-
Low-density lipoprotein
- LM
-
Lipid mediators
- LOX
-
Lipoxygenase
- LTB4
-
Leukotriene B4
- MAR1
-
Nuclear matrix-associated protein MAR1
- MS
-
Mass spectrometry
- MUFA
-
Monounsaturated fatty acid
- NS
-
Not significant
- PD1
-
Programmed cell death protein 1
- PGE2
-
Prostaglandin E2
- PnK
-
Pronokal
- PUFA
-
Polyunsaturated fatty acid
- RBC
-
Red blood cell
- RVD2
-
7(S), 16(R), 17(S)-resolvin D2
- SD
-
Standard deviation
- SFA
-
Saturated fatty acid
- SPM
-
Proresolution lipid mediators
- TNF
-
Tumor necrosis factor
- TXB2
-
T-box transcription factor
- VLKD
-
Very low-calorie ketogenic diet
- WC
-
Waist circumference
Rights and permissions
About this article
Cite this article
de Luis, D., Domingo, J.C., Izaola, O. et al. Effect of DHA supplementation in a very low-calorie ketogenic diet in the treatment of obesity: a randomized clinical trial. Endocrine 54, 111–122 (2016). https://doi.org/10.1007/s12020-016-0964-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-0964-z