Abstract
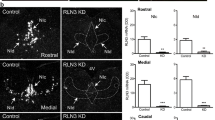
Prolactin is an anterior pituitary hormone necessary for fertility, pregnancy maintenance, lactation, and aspects of maternal behavior. In rodents, there is a surge of prolactin on the afternoon of proestrus, and a semi-circadian pattern of prolactin surges during early pregnancy, with a diurnal and nocturnal surge every day. Both of these patterns can be replicated in ovariectomized rats. A prior study demonstrated that central antagonism of κ-opioid receptors, the target of dynorphin, largely abolished the nocturnal prolactin surge in pregnant rats. We build on this to determine whether dynorphin, perhaps from the arcuate population that co-express kisspeptin, neurokinin B, and dynorphin (KNDy neurons), also contributes to the estradiol- or cervical stimulation-induced surges in ovariectomized rats. Ovariectomized rats were treated with either estradiol or cervical stimulation to induce prolactin surge(s). Blood samples were taken around the expected surge time to determine the effect of either acute κ-opioid receptor antagonism or previous chemical ablation of the KNDy population on prolactin levels. Dynorphin antagonism does significantly disrupt the nocturnal prolactin surge, but it does not contribute to the estradiol-induced surge. Chemical ablation of KNDy neurons had opposite effects; ablation of 40 % of the KNDy neurons had no impact on the nocturnal prolactin surge, while a somewhat larger ablation significantly reduced the size of the estradiol-induced surge. We conclude that dynorphin is likely a controlling factor for the nocturnal surge induced by cervical stimulation, and that other KNDy neuron products must play a role in the estradiol-induced surge.






Similar content being viewed by others
Abbreviations
- CS:
-
Cervical stimulation
- DA:
-
Dopamine
- DOPAC:
-
3,4-Dihydroxyphenylacetic acid
- E2 :
-
Estradiol
- EDTA:
-
Ethylenediaminetetraacetic acid
- HPLC:
-
High performance liquid chromatography
- KNDy:
-
Kisspeptin, neurokinin B, & dynorphin
- KOR:
-
κ opioid receptor
- LH:
-
Luteinizing hormone
- nor-BNI:
-
Norbinaltorphimine
- OVE:
-
Ovariectomized with estrogen replacement
- OVX:
-
Ovariectomized
- PBS:
-
Phospho-buffered saline
- RIA:
-
Radioimmunoassay
- TH:
-
Tyrosine hydroxylase
References
M.E. Freeman, B. Kanicska, A. Lerant, G.M. Nagy, Prolactin: structure, function and regulation of secretion. Physiol. Rev. 80(4), 1523–1631 (2000)
M.E. Freeman, Neuroendocrine control of the ovarian cycle of the rat, in Knobil and Neill’s Physiology of Reproduction, ed. by J.D. Neill (Academic Press, San Diego, 2006), pp. 2327–2388
M.E. Freeman, M.S. Smith, S.J. Nazian, J.D. Neill, Ovarian and hypothalamic control of daily surges of prolactin secretion during pseudopregnancy in the rat. Endocrinology 94, 875–882 (1974)
P. Kadioglu, A.S. Yalin, O. Tiryakioglu, N. Gazioglu, G. Oral, O. Sanli, K. Onem, A. Kadioglu, Sexual dysfunction in women with hyperprolactinemia: a pilot study report. J. Urol. 174, 1921–1925 (2005)
M. Egli, B. Leeners, T.H.C. Kruger, Prolactin secretion patterns: basic mechanisms and clinical implications for reproduction. Reproduction 140, 643–645 (2010)
C.M. Larsen, D.R. Grattan, Prolactin-induced mitogenesis in the subventricular zone of the maternal brain during early pregnancy is essential for normal postpartum behavioral responses in the mother. Endocrinology 151(8), 3805–3814 (2010)
T.H. Kruger, B. Leeners, E. Naegeli, S. Schmidlin, M. Schedlowski, U. Hartmann, M. Egli, Prolactin secretory rhythm in women: immediate and long term alterations after sexual contact. Hum. Reprod. 27(4), 1139–1143 (2012)
D.R. Grattan, I.C. Kokay, Prolactin: a pleiotropic neuroendocrine hormone. J. Neuroendocrinol. 20, 752–763 (2008)
D.J. Sirinathsinghji, A.R. Audsley, Endogenous opioid peptides participate in the modulation of prolactin release in response to cervicovaginal stimulation in the female rat. Endocrinology 117(2), 549–556 (1985)
C.A. Sagrillo, J.L. Voogt, Endogenous opioids mediate the nocturnal prolactin surge in the pregnant rat. Endocrinology 129(2), 925–930 (1991)
Y. Hou, J.L. Voogt, Effects of naloxone infusion on nocturnal prolactin secretion and Fos/FRA expression in pregnant rats. Endocrine 10(2), 145–152 (1999)
B. Zhang, Y. Hou, J.L. Voogt, Effects of opioid antagonism on prolactin secretion and c-Fos/TH expression during lactation in rats. Endocrine 25(2), 131–136 (2004)
Z.B. Andrews, D.R. Grattan, Opioid receptor subtypes involved in the regulation of prolactin secretion during pregnancy and lactation. J. Neuroendocrinol. 15, 227–236 (2003)
C.V. Helena, N. Toporikova, B. Kalil, A.M. Stathopoulos, R.O. Carolino, J.A. Anselmo-Franci, R. Bertram, KNDy neurons modulate the steroid-induced luteinizing hormone surge in ovariectomized rats. Endocrinology 156(11), 4200–4213 (2015)
X.F. Li, J.S. Kinsey-Jones, Y. Cheng, A.M.I. Knox, Y. Lin, N.A. Petrou, A. Roseweir, S.L. Lightman, S.R. Milligan, R.P. Millar, K.T. O’Byrne, Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS ONE 4(12), e8334 (2009)
V.M. Navarro, M.L. Gottsh, C. Chavkin, H. Okamura, D. Clifton, R.A. Steiner, Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci. 29(38), 11859–11866 (2009)
M. Kirilov, J. Clarkson, X. Liu, J. Roa, P. Campos, R. Porteous, G. Schutz, A.E. Herbison, Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nat. Commun. 4, 2492 (2013)
K.E. Beale, J.S. Kinsey-Jones, J.V. Gardiner, E.K. Harrison, El Thompson, M.H. Hu, M.L. Sleeth et al., The physiological role of arcuate kisspeptin neurons in the control of reproductive function in female rats. Endocrinology 155(3), 1091–1098 (2014)
R.E. Szawka, A.B. Rieiro, C.M. Leite, C.V.V. Helena, C.R. Franci, G.M. Anderson, G.E. Hoffman, J.A. Anselmo-Franci, Kisspeptin regulates prolactin release through hypothalamic dopaminergic neurons. Endocrinology 151(7), 3247–3257 (2014)
J.J. Wagner, R.M. Caudle, C. Chavkin, Kappa-opioids decrease excitatory transmission in the dentate gyrus of the guinea pig hypothalamus. J. Neurosci. 12(1), 132–141 (1992)
A. Mansour, C.A. Fox, S. Burke, F. Meng, R.C. Thompson, H. Akil, S.J. Watson, Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J. Comp. Neurol. 350(3), 412–438 (1994)
M.D. Fitzsimmons, J.A. Olschowka, S.J. Wiegand, G.E. Hoffman, Interaction of opioid peptide-containing terminals with dopaminergic perikarya in the rat hypothalamus. Brain Res. 581(1), 10–18 (1992)
X. Zhang, A.N. van den Pol, Dopamine/tyrosine hydroxylase neurons of the hypothalamic arcuate nucleus release GABA, communicate with dopaminergic and other arcuate neurons, and respond to dyrnorphin, met-enkephalin, and oxytocin. J. Neurosci. 35(45), 14966–14982 (2015)
M.A. Mittelman-Smith, H. Williams, S.J. Krajewski-Hall, J. Lai, P. Ciofi, N.T. McMullen, N.E. Rance, Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology 153(6), 2800–2812 (2012)
M.F. Cordellini, G. Piazzetta, K.C. Pinto, A.M. Delattre, F. Matheussi, R.O.G. Carolino, R.E. Szawka, J.A. Anselmo-Franci, A.C. Ferraz, Effect of different doses of estrogen on the nigrostriatal dopaminergic system in two 6-hydroxydopamine-induced lesion models of Parkinson’s disease. Neurochem. Res. 36(6), 955–961 (2011)
K.J. Lookingland, H.D. Jarry, K.E. Moore, The metabolism of dopamine in the median eminence reflects the activity of tuberoinfundibular neurons. Brain Res. 419(1–2), 303–310 (1987)
M.C. Burke, P.A. Letts, S.J. Krajweski, N.E. Rance, Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J. Comp. Neurol. 498, 712–726 (2006)
C.F. Witty, P.W. Weems, R.L. Goodman, L.M. Coolen, M.N. Lehman, Kappa opioid receptor is present within a majority of KNDy neurons in the ewe, in Neuroscience Meeting Planner, NN33, (Society for Neuroscience, Washington, DC, 2014) Online
P.W. Weems, C.F. Witty, M. Amstalden, L.M. Coolen, R.L. Goodman, M.N. Lehman, Kappa opioid receptor is co-localized in GnRH and KNDy cells in the female ovine and rodent brains. (submitted)
A.M. DePaoli, K.M. Hurley, K. Yasada, T. Reisine, G. Bell, Distribution of kappa opioid receptor mRNA in adult mouse brain: an in situ hybridization histochemistry study. Mol. Cell. Neurosci. 5(4), 327–335 (1994)
Y.J. Wang, K. Rasakham, P. Huang, D. Chudnovskaya, A. Cowan, L.Y. Liu-Chen, Sex difference in κ-opioid receptor (KOPR)-mediated behaviors, brain region KOPR level and KOPR-mediated guanosine 5′-O-(3-[35S] thiotriphosphate) binding in the guinea pig. J. Pharmacol. Exp. Ther. 339(2), 438–450 (2011)
N. Maolood, B. Meister, Dynorphin in pro-opiomelanocortin neurons of the hypothalamic arcuate nucleus. Neuroscience 154(3), 1121–1131 (2008)
J.T. Smith, M.J. Cunningham, E.F. Rissman, D.K. Clifton, R.A. Steiner, Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146(9), 3686–3692 (2005)
P. Mostari, N. Ieda, C. Deura, S. Minabe, S. Yamada, Y. Uenoyama, K. Maeda, H. Tsukamura, Dynorphin-kappa opioid receptor signaling partly mediates estrogen negative feedback effect on LH pulses in female rats. J. Reprod. Dev. 59(3), 266–272 (2013)
Q. Zhang, R.V. Gallo, Effect of prodynorphin-derived opioid peptides on the ovulatory luteinizing hormone surge in the proestrous rat. Endocrine 18(1), 27–32 (2002)
Q. Zhang, R.V. Gallo, Presence of κ-opioid tone at the onset of the ovulatory luteinizing hormone surge in the proestrous rat. Brain Res. 290, 135–139 (2003)
E.J. Wagner, J. Manzanares, K.E. Moore, K.L. Lookingland, Neurochemical evidence that estrogen-induced suppression of kappa-opioid-receptor-mediated regulation of tuberoinfundibular dopaminergic neurons is prolactin-independent. Neuroendocrinology 59, 197–201 (1994)
Acknowledgments
We thank Kim Hughes for helpful discussions, Frank Johnson for the use of his microscope, Charles Badland for assistance with figure preparation, and Lique Coolen for the antibody protocol. We also thank Frank Nenninger and Jose Arias-Cristancho, for generous assistance with surgeries, animal care, and blood sampling. This work was partially supported by the National Institutes of Health grant DK-43200.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None of the authors report any conflict of interest.
Human and Animal Rights
Animal procedures were approved by the Florida State University Animal Care and Use Committee.
Rights and permissions
About this article
Cite this article
Stathopoulos, A.M., Helena, C.V., Cristancho-Gordo, R. et al. Influence of dynorphin on estradiol- and cervical stimulation-induced prolactin surges in ovariectomized rats. Endocrine 53, 585–594 (2016). https://doi.org/10.1007/s12020-016-0938-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-0938-1