Abstract
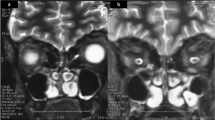
One of the challenging issues in patients with complex problems is that the various diseases and their treatment can influence each other and present unusual hurdles in management. We investigated one such complex case. A 34-year-old XY male presented with azoospermia, detected on semen analysis for pre-orchidectomy sperm banking. He had a 20-year history of gender dysphoria and bilateral breast swelling. The patient suffered a deep vein thrombosis at the age of 19 years. Examination confirmed clinical features of Kallmann syndrome including unilateral cryptorchidism, micropenis, congenital anosmia, and bimanual synkinesis (mirror movements), with reduced serum testosterone and normal gonadotropin levels demonstrating hypogonadotropic hypogonadism. MRI showed missing olfactory bulbs. Osteopenia and reduced vitamin D levels of 21 nmol/L were identified. He was found to harbor a heterozygous factor-V-Leiden mutation. The genetic basis of Kallmann syndrome remains unknown: his screening tests were negative for mutations in CHD7, FGF8, FGFR1, GNRH1, GNRHR, HS6ST1, KAL1, KISS1R, KISS1, NELF, PROK2, PROKR2, TAC3, and TACR3. The patient initially declined testosterone therapy with a view to undergo gender reassignment. Over the next 2 years, the patient experienced recurrent episodes of weakness and paresthesia, associated with classical MRI appearances of multiple sclerosis-related demyelination in the spinal cord and brain. Although it was difficult to elucidate an association between the patient’s gender dysphoria and untreated congenital hypogonadism, his desire to become female together with his co-existing thrombophilia, presented challenges to the administration of hormone treatment. Furthermore, we have considered an association between multiple sclerosis and hypogonadotropic hypogonadism.



Similar content being viewed by others
References
Royal College of Psychiatrists: Good practice guidelines for the assessment and treatment of adults with gender dysphoria (2013), http://www.rcpsych.ac.uk/files/pdfversion/CR181.pdf. Accessed 30 May 2014
W.C. Hembree, P. Cohen-Kettenis, H.A. de Delemarre-van Waal, L.J. Gooren, W.J. Meyer, N.P. Spack, V. Tangpricha, V.M. Montori, E. Society, Endocrine treatment of transsexual persons: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 94(9), 3132–3154 (2009). doi:10.1210/jc.2009-0345
WPATH, Standards of care for the health of transsexual, transgender, and gender-nonconforming people. Int. J. Transgend. 13(4), 165–232 (2012)
S. Melmed, K. Polonsky, P. Larsen, H. Kronenberg, Williams Textbook of Endocrinology, 12th edn. (Saunders/Elsevier, Philadelphia, 2011)
N. Pitteloud, R. Quinton, S. Pearce, T. Raivio, J. Acierno, A. Dwyer, L. Plummer, V. Hughes, S. Seminara, Y.Z. Cheng, W.P. Li, G. Maccoll, A.V. Eliseenkova, S.K. Olsen, O.A. Ibrahimi, F.J. Hayes, P. Boepple, J.E. Hall, P. Bouloux, M. Mohammadi, W. Crowley, Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J. Clin. Invest. 117(2), 457–463 (2007). doi:10.1172/JCI29884
H. Miraoui, A.A. Dwyer, G.P. Sykiotis, L. Plummer, W. Chung, B. Feng, A. Beenken, J. Clarke, T.H. Pers, P. Dworzynski, K. Keefe, M. Niedziela, T. Raivio, W.F. Crowley, S.B. Seminara, R. Quinton, V.A. Hughes, P. Kumanov, J. Young, M.A. Yialamas, J.E. Hall, G. Van Vliet, J.P. Chanoine, J. Rubenstein, M. Mohammadi, P.S. Tsai, Y. Sidis, K. Lage, N. Pitteloud, Mutations in FGF17, IL17RD, DUSP6, SPRY4, and FLRT3 are identified in individuals with congenital hypogonadotropic hypogonadism. Am. J. Hum. Genet. 92(5), 725–743 (2013). doi:10.1016/j.ajhg.2013.04.008
A. de Maestre San Juan, Falta total de los nervios olfactorios con anosmia en un individuo en quien existía una atrofia congénita de los testículos y el miembro viril. El Siglo Médico 131, 211 (1856)
F. Kallmann, W. Schoenfeld, S. Barrera, The genetic aspects of primary eunuchoidism. Am. J. Ment. Defic. 48, 203–236 (1944)
E.M. Laitinen, K. Vaaralahti, J. Tommiska, E. Eklund, M. Tervaniemi, L. Valanne, T. Raivio, Incidence, phenotypic features and molecular genetics of Kallmann syndrome in Finland. Orphanet. J. Rare Dis. 6, 41 (2011). doi:10.1186/1750-1172-6-41
D. Garcia-Gonzalez, V. Murcia-Belmonte, D. Clemente, F. De Castro, Olfactory system and demyelination. Anat. Rec. 296(9), 1424–1434 (2013). doi:10.1002/ar.22736
C. Dodé, J.P. Hardelin, Kallmann syndrome. Eur. J. Hum. Genet. 17(2), 139–146 (2009). doi:10.1038/ejhg.2008.206
J. Tommiska, J. Känsäkoski, P. Christiansen, N. Jørgensen, J.G. Lawaetz, A. Juul, T. Raivio, Genetics of congenital hypogonadotropic hypogonadism in Denmark. Eur. J. Med. Genet. 57(7), 345–348 (2014). doi:10.1016/j.ejmg.2014.04.002
K. Jarzabek, S. Wolczynski, R. Lesniewicz, G. Plessis, M.L. Kottler, Evidence that FGFR1 loss-of-function mutations may cause variable skeletal malformations in patients with Kallmann syndrome. Adv. Med. Sci. 57(2), 314–321 (2012). doi:10.2478/v10039-012-0036-4
T. Raivio, M. Avbelj, M.J. McCabe, C.J. Romero, A.A. Dwyer, J. Tommiska, G.P. Sykiotis, L.C. Gregory, D. Diaczok, V. Tziaferi, M.W. Elting, R. Padidela, L. Plummer, C. Martin, B. Feng, C. Zhang, Q.Y. Zhou, H. Chen, M. Mohammadi, R. Quinton, Y. Sidis, S. Radovick, M.T. Dattani, N. Pitteloud, Genetic overlap in Kallmann syndrome, combined pituitary hormone deficiency, and septo-optic dysplasia. J. Clin. Endocrinol. Metab. 97(4), E694–E699 (2012). doi:10.1210/jc.2011-2938
D. Büchter, H.M. Behre, S. Kliesch, E. Nieschlag, Pulsatile GnRH or human chorionic gonadotropin/human menopausal gonadotropin as effective treatment for men with hypogonadotropic hypogonadism: a review of 42 cases. Eur. J. Endocrinol. 139(3), 298–303 (1998)
R. Quinton, H.K. Cheow, D.J. Tymms, P.M. Bouloux, F.C. Wu, H.S. Jacobs, Kallmann’s syndrome: is it always for life? Clin. Endocrinol. 50(4), 481–485 (1999)
T. Raivio, J. Falardeau, A. Dwyer, R. Quinton, F.J. Hayes, V.A. Hughes, L.W. Cole, S.H. Pearce, H. Lee, P. Boepple, W.F. Crowley, N. Pitteloud, Reversal of idiopathic hypogonadotropic hypogonadism. N. Engl. J. Med. 357(9), 863–873 (2007). doi:10.1056/NEJMoa066494
V.F. Sidhoum, Y.M. Chan, M.F. Lippincott, R. Balasubramanian, R. Quinton, L. Plummer, A. Dwyer, N. Pitteloud, F.J. Hayes, J.E. Hall, K.A. Martin, P.A. Boepple, S.B. Seminara, Reversal and relapse of hypogonadotropic hypogonadism: resilience and fragility of the reproductive neuroendocrine system. J. Clin. Endocrinol. Metab. 99(3), 861–870 (2014). doi:10.1210/jc.2013-2809
A. Bauman, Markedly delayed puberty or Kallmann’s syndrome variant. J. Androl. 7(4), 224–227 (1986)
A. Kadva, W.L. Di, O. Djahanbakhch, J. Monson, R. Silman, Evidence for the Bauman variant in Kallmann’s syndrome. Clin. Endocrinol. 44(1), 103–110 (1996)
H. Burger-Prinz, H. Albrecht, H. Giese, Zur Phanomenologie des Transvestitismus bei Mannern [On phenomenology of the transvestism in men] (Enke, Stuttgart, 1966)
P.-A. Fischer, Korperliche Befunde bei psychosexuellen Storungen, in Die Sexualitat des Menschen [Human Sexuality], ed. by H. Giese (Enke, Stuttgart, 1971), pp. 940–955
B. Meyenburg, V. Sigusch, Kallmann’s syndrome and transsexualism. Arch. Sex. Behav. 30(1), 75–81 (2001)
Jopp, D.A., Keys, C.B.: Diagnostic overshadowing reviewed and reconsidered. Am J Ment Retard 106(5), 416-433 (2001). doi:10.1352/0895-8017(2001)1062.0.CO;2
K.S. Kim, J. Kim, Disorders of sex development. Korean J. Urol. 53(1), 1–8 (2012). doi:10.4111/kju.2012.53.1.1
A. Marchiori, L. Mosena, M.H. Prins, P. Prandoni, The risk of recurrent venous thromboembolism among heterozygous carriers of factor V Leiden or prothrombin G20210A mutation. A systematic review of prospective studies. Haematologica 92(8), 1107–1114 (2007)
M.R. Aboud, D.D. Ma, Increased incidence of venous thrombosis in patients with shortened activated partial thromboplastin times and low ratios for activated protein C resistance. Clin. Lab. Haematol. 23(6), 411–416 (2001)
M. Sartori, L. Migliaccio, E. Favaretto, G. Palareti, B. Cosmi, Two years outcome of isolated distal deep vein thrombosis. Thromb. Res. 134(1), 36–40 (2014). doi:10.1016/j.thromres.2014.03.033
P. Angelova, A. Momchilova, D. Petkova, G. Staneva, R. Pankov, Z. Kamenov, Testosterone replacement therapy improves erythrocyte membrane lipid composition in hypogonadal men. Aging Male 15(3), 173–179 (2012). doi:10.3109/13685538.2012.693550
C.J. Glueck, N. Goldenberg, S. Budhani, D. Lotner, C. Abuchaibe, M. Gowda, T. Nayar, N. Khan, P. Wang, Thrombotic events after starting exogenous testosterone in men with previously undiagnosed familial thrombophilia. Transl. Res. 158(4), 225–234 (2011). doi:10.1016/j.trsl.2011.06.003
FDA: FDA adding general warning to testosterone products about potential for venous blood clots. (2014) http://www.fda.gov/Drugs/DrugSafety/ucm401746.htm. Accessed 16 Jun 2014
A. Gaby, Multiple Sclerosis. Glob Adv. Health Med. 2(1), 50–56 (2013). doi:10.7453/gahmj.2013.2.1.009
I.S. Mackenzie, S.V. Morant, G.A. Bloomfield, T.M. MacDonald, J. O’Riordan, Incidence and prevalence of multiple sclerosis in the UK 1990-2010: a descriptive study in the General Practice Research Database. J. Neurol. Neurosurg. Psychiatry 85(1), 76–84 (2014). doi:10.1136/jnnp-2013-305450
S. Sawcer, G. Hellenthal, M. Pirinen, C.C. Spencer, N.A. Patsopoulos, L. Moutsianas, A. Dilthey, Z. Su, C. Freeman, S.E. Hunt, S. Edkins, E. Gray, D.R. Booth, S.C. Potter, A. Goris, G. Band, A.B. Oturai, A. Strange, J. Saarela, C. Bellenguez, B. Fontaine, M. Gillman, B. Hemmer, R. Gwilliam, F. Zipp, A. Jayakumar, R. Martin, S. Leslie, S. Hawkins, E. Giannoulatou, S. D’alfonso, H. Blackburn, F. Martinelli Boneschi, J. Liddle, H.F. Harbo, M.L. Perez, A. Spurkland, M.J. Waller, M.P. Mycko, M. Ricketts, M. Comabella, N. Hammond, I. Kockum, O.T. McCann, M. Ban, P. Whittaker, A. Kemppinen, P. Weston, C. Hawkins, S. Widaa, J. Zajicek, S. Dronov, N. Robertson, S.J. Bumpstead, L.F. Barcellos, R. Ravindrarajah, R. Abraham, L. Alfredsson, K. Ardlie, C. Aubin, A. Baker, K. Baker, S.E. Baranzini, L. Bergamaschi, R. Bergamaschi, A. Bernstein, A. Berthele, M. Boggild, J.P. Bradfield, D. Brassat, S.A. Broadley, D. Buck, H. Butzkueven, R. Capra, W.M. Carroll, P. Cavalla, E.G. Celius, S. Cepok, R. Chiavacci, F. Clerget-Darpoux, K. Clysters, G. Comi, M. Cossburn, I. Cournu-Rebeix, M.B. Cox, W. Cozen, B.A. Cree, A.H. Cross, D. Cusi, M.J. Daly, E. Davis, P.I. de Bakker, M. Debouverie, M.B. D’hooghe, K. Dixon, R. Dobosi, B. Dubois, D. Ellinghaus, I. Elovaara, F. Esposito, C. Fontenille, S. Foote, A. Franke, D. Galimberti, A. Ghezzi, J. Glessner, R. Gomez, O. Gout, C. Graham, S.F. Grant, F.R. Guerini, H. Hakonarson, P. Hall, A. Hamsten, H.P. Hartung, R.N. Heard, S. Heath, J. Hobart, M. Hoshi, C. Infante-Duarte, G. Ingram, W. Ingram, T. Islam, M. Jagodic, M. Kabesch, A.G. Kermode, T.J. Kilpatrick, C. Kim, N. Klopp, K. Koivisto, M. Larsson, M. Lathrop, J.S. Lechner-Scott, M.A. Leone, V. Leppä, U. Liljedahl, I.L. Bomfim, R.R. Lincoln, J. Link, J. Liu, A.R. Lorentzen, S. Lupoli, F. Macciardi, T. Mack, M. Marriott, V. Martinelli, D. Mason, J.L. McCauley, F. Mentch, I.L. Mero, T. Mihalova, X. Montalban, J. Mottershead, K.M. Myhr, P. Naldi, W. Ollier, A. Page, A. Palotie, J. Pelletier, L. Piccio, T. Pickersgill, F. Piehl, S. Pobywajlo, H.L. Quach, P.P. Ramsay, M. Reunanen, R. Reynolds, J.D. Rioux, M. Rodegher, S. Roesner, J.P. Rubio, I.M. Rückert, M. Salvetti, E. Salvi, A. Santaniello, C.A. Schaefer, S. Schreiber, C. Schulze, R.J. Scott, F. Sellebjerg, K.W. Selmaj, D. Sexton, L. Shen, B. Simms-Acuna, S. Skidmore, P.M. Sleiman, C. Smestad, P.S. Sørensen, H.B. Søndergaard, J. Stankovich, R.C. Strange, A.M. Sulonen, E. Sundqvist, A.C. Syvänen, F. Taddeo, B. Taylor, J.M. Blackwell, P. Tienari, E. Bramon, A. Tourbah, M.A. Brown, E. Tronczynska, J.P. Casas, N. Tubridy, A. Corvin, J. Vickery, J. Jankowski, P. Villoslada, H.S. Markus, K. Wang, C.G. Mathew, J. Wason, C.N. Palmer, H.E. Wichmann, R. Plomin, E. Willoughby, A. Rautanen, J. Winkelmann, M. Wittig, R.C. Trembath, J. Yaouanq, A.C. Viswanathan, H. Zhang, N.W. Wood, R. Zuvich, P. Deloukas, C. Langford, A. Duncanson, J.R. Oksenberg, M.A. Pericak-Vance, J.L. Haines, T. Olsson, J. Hillert, A.J. Ivinson, P.L. De Jager, L. Peltonen, G.J. Stewart, D.A. Hafler, S.L. Hauser, G. McVean, P. Donnelly, A. Compston, I.M.S.G. Consortium, 2, W.T.C.C.C.: Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476(7359), 214–219 (2011). doi:10.1038/nature10251
C. O’Gorman, R. Lin, J. Stankovich, S.A. Broadley, Modelling genetic susceptibility to multiple sclerosis with family data. Neuroepidemiology 40(1), 1–12 (2013). doi:10.1159/000341902
R. Manara, A. Salvalaggio, A. Favaro, V. Palumbo, V. Citton, A. Elefante, A. Brunetti, F. Di Salle, G. Bonanni, A.A. Sinisi, Group K.S.N.S., Brain changes in Kallmann syndrome. Am. J. Neuroradiol. 35(9), 1700–1706 (2014). doi:10.3174/ajnr.A3946
R. Quinton, V.M. Duke, A. Robertson, J.M. Kirk, G. Matfin, P.A. de Zoysa, C. Azcona, G.S. MacColl, H.S. Jacobs, G.S. Conway, M. Besser, R.G. Stanhope, P.M. Bouloux, Idiopathic gonadotrophin deficiency: genetic questions addressed through phenotypic characterization. Clin. Endocrinol. 55(2), 163–174 (2001)
D. Clemente, M.C. Ortega, F.J. Arenzana, F. de Castro, FGF-2 and Anosmin-1 are selectively expressed in different types of multiple sclerosis lesions. J. Neurosci. 31(42), 14899–14909 (2011). doi:10.1523/JNEUROSCI.1158-11.2011
J. Antel, S. Antel, Z. Caramanos, D.L. Arnold, T. Kuhlmann, Primary progressive multiple sclerosis: part of the MS disease spectrum or separate disease entity? Acta Neuropathol. 123(5), 627–638 (2012). doi:10.1007/s00401-012-0953-0
P. Iwanowski, J. Losy, Immunological differences between classical phenotypes of multiple sclerosis. J. Neurol. Sci. (2015). doi:10.1016/j.jns.2014.12.035
B.F. Bebo, J.C. Schuster, A.A. Vandenbark, H. Offner, Androgens alter the cytokine profile and reduce encephalitogenicity of myelin-reactive T cells. J. Immunol. 162(1), 35–40 (1999)
B.F. Bebo, E. Zelinka-Vincent, G. Adamus, D. Amundson, A.A. Vandenbark, H. Offner, Gonadal hormones influence the immune response to PLP 139-151 and the clinical course of relapsing experimental autoimmune encephalomyelitis. J. Neuroimmunol. 84(2), 122–130 (1998)
S. Kim, R.R. Voskuhl, Decreased IL-12 production underlies the decreased ability of male lymph node cells to induce experimental autoimmune encephalomyelitis. J. Immunol. 162(9), 5561–5568 (1999)
M. Dalal, S. Kim, R.R. Voskuhl, Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J. Immunol. 159(1), 3–6 (1997)
S.M. Liva, R.R. Voskuhl, Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J. Immunol. 167(4), 2060–2067 (2001)
N.L. Sicotte, B.S. Giesser, V. Tandon, R. Klutch, B. Steiner, A.E. Drain, D.W. Shattuck, L. Hull, H.J. Wang, R.M. Elashoff, R.S. Swerdloff, R.R. Voskuhl, Testosterone treatment in multiple sclerosis: a pilot study. Arch. Neurol. 64(5), 683–688 (2007). doi:10.1001/archneur.64.5.683
F. Kurth, E. Luders, N.L. Sicotte, C. Gaser, B.S. Giesser, R.S. Swerdloff, M.J. Montag, R.R. Voskuhl, A. Mackenzie-Graham, Neuroprotective effects of testosterone treatment in men with multiple sclerosis. Neuroimage. Clin. 4, 454–460 (2014). doi:10.1016/j.nicl.2014.03.001
Acknowledgments
We are grateful to the patient for allowing us to share the history and data of his diseases.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Renukanthan, A., Quinton, R., Turner, B. et al. Kallmann syndrome patient with gender dysphoria, multiple sclerosis, and thrombophilia. Endocrine 50, 496–503 (2015). https://doi.org/10.1007/s12020-015-0562-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-015-0562-5