Abstract
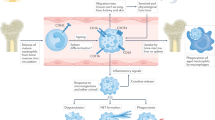
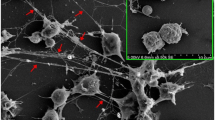
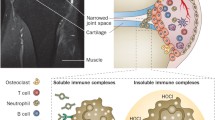
Systemic rheumatic diseases are a heterogeneous group of disorders characterized by profound immune dysregulation. Recent discoveries have led to a significant resurgence of interest in neutrophils as shapers of immune dysregulation and as triggers of organ damage in rheumatic diseases. Neutrophils contribute to the initiation, promotion, and perpetuation of immune dysregulation through a variety of mechanisms including synthesis of proinflammatory cytokines, direct tissue damage through degranulation and synthesis of reactive oxygen species, and the formation of neutrophil extracellular traps (NETs). The identification of a subset of proinflammatory neutrophils, the low-density granulocytes (LDGs), which promote Th1 responses and cause endothelial dysfunction, has further strengthened the pathogenic role of neutrophils in various rheumatic diseases. The presence of autoantibodies targeting molecules commonly expressed in neutrophils suggests that neutrophils, particularly NETs, may be a source of autoantigens. An imbalance between NET formation and degradation, which leads to increased NET levels in the circulation and tissues, could enhance the exposure of the immune system to modified autoantigens, promote vascular disease, and increase tissue damage. This review will present an overview of recent advances in our understanding of how neutrophil dysregulation modulates the innate and adaptive immune responses in systemic rheumatic diseases and their putative contributions to pathogenicity. Understanding the potential pathogenic role of neutrophil dysregulation may provide better molecular candidates for therapeutic targeting, and ultimately promote improvements in the clinical outcomes in rheumatic diseases.



Similar content being viewed by others
References
Goldblatt F, O’Neill SG (2013) Clinical aspects of autoimmune rheumatic diseases. Lancet 382(9894):797–808. https://doi.org/10.1016/S0140-6736(13)61499-3
Deane KD, El-Gabalawy H (2014) Pathogenesis and prevention of rheumatic disease: focus on preclinical RA and SLE. Nat Rev Rheumatol 10(4):212–228. https://doi.org/10.1038/nrrheum.2014.6
Tsokos GC (2020) Autoimmunity and organ damage in systemic lupus erythematosus. Nat Immunol 21(6):605–614. https://doi.org/10.1038/s41590-020-0677-6
Denny MF, Yalavarthi S, Zhao W et al (2010) A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol 184(6):3284–3297. https://doi.org/10.4049/jimmunol.0902199
Brinkmann V, Zychlinsky A (2007) Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol 5(8):577–582. https://doi.org/10.1038/nrmicro1710
Apel F, Zychlinsky A, Kenny EF (2018) The role of neutrophil extracellular traps in rheumatic diseases. Nat Rev Rheumatol 14(8):467–475. https://doi.org/10.1038/s41584-018-0039-z
Mutua V, Gershwin LJ (2020) A review of neutrophil extracellular traps (NETs) in disease: potential anti-NETs therapeutics. Clin Rev Allergy Immunol. https://doi.org/10.1007/s12016-020-08804-7
Mantovani A, Cassatella MA, Costantini C et al (2011) Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 11(8):519–531. https://doi.org/10.1038/nri3024
Ley K, Hoffman HM, Kubes P et al (2018) Neutrophils: new insights and open questions. Sci Immunol 3 (30). https://doi.org/10.1126/sciimmunol.aat4579
van Gisbergen KP, Sanchez-Hernandez M, Geijtenbeek TB et al (2005) Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J Exp Med 201(8):1281–1292. https://doi.org/10.1084/jem.20041276
Pelletier M, Maggi L, Micheletti A et al (2010) Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 115(2):335–343. https://doi.org/10.1182/blood-2009-04-216085
Puga I, Cols M, Barra CM et al (2011) B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol 13(2):170–180. https://doi.org/10.1038/ni.2194
Huard B, McKee T, Bosshard C et al (2008) APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J Clin Invest 118(8):2887–2895. https://doi.org/10.1172/JCI33760
Hacbarth E, Kajdacsy-Balla A (1986) Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum 29(11):1334–1342. https://doi.org/10.1002/art.1780291105
Bennett L, Palucka AK, Arce E et al (2003) Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med 197(6):711–723. https://doi.org/10.1084/jem.20021553
Liu Y, Xia C, Chen J et al (2020) Elevated circulating pro-inflammatory low-density granulocytes in adult-onset Still’s disease. Rheumatology (Oxford). https://doi.org/10.1093/rheumatology/keaa324
Marwick JA, Mills R, Kay O et al (2018) Neutrophils induce macrophage anti-inflammatory reprogramming by suppressing NF-kappaB activation. Cell Death Dis 9(6):665. https://doi.org/10.1038/s41419-018-0710-y
Miles K, Clarke DJ, Lu W et al (2009) Dying and necrotic neutrophils are anti-inflammatory secondary to the release of alpha-defensins. J Immunol 183(3):2122–2132. https://doi.org/10.4049/jimmunol.0804187
Peiseler M, Kubes P (2019) More friend than foe: the emerging role of neutrophils in tissue repair. J Clin Invest 129(7):2629–2639. https://doi.org/10.1172/JCI124616
Theilgaard-Monch K, Knudsen S, Follin P et al (2004) The transcriptional activation program of human neutrophils in skin lesions supports their important role in wound healing. J Immunol 172(12):7684–7693. https://doi.org/10.4049/jimmunol.172.12.7684
Rhys HI, Dell’Accio F, Pitzalis C et al (2018) Neutrophil microvesicles from healthy control and rheumatoid arthritis patients prevent the inflammatory activation of macrophages. EBioMedicine 29:60–69. https://doi.org/10.1016/j.ebiom.2018.02.003
Brinkmann V, Reichard U, Goosmann C et al (2004) Neutrophil extracellular traps kill bacteria. Science 303(5663):1532–1535. https://doi.org/10.1126/science.1092385
Boeltz S, Amini P, Anders HJ et al (2019) To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ 26(3):395–408. https://doi.org/10.1038/s41418-018-0261-x
Li P, Li M, Lindberg MR et al (2010) PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med 207(9):1853–1862. https://doi.org/10.1084/jem.20100239
Chen KW, Monteleone M, Boucher D et al (2018) Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci Immunol 3 (26). https://doi.org/10.1126/sciimmunol.aar6676
Sollberger G, Choidas A, Burn GL et al (2018) Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol 3 (26). https://doi.org/10.1126/sciimmunol.aar6689
Wang X, Blanco LP, Carmona-Rivera C et al (2020) Gasdermin D modulates murine lupus and its associated organ damage. Arthritis Rheumatol. https://doi.org/10.1002/art.41444
Kahlenberg JM, Carmona-Rivera C, Smith CK et al (2013) Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol 190(3):1217–1226. https://doi.org/10.4049/jimmunol.1202388
Lande R, Gregorio J, Facchinetti V et al (2007) Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449(7162):564–569. https://doi.org/10.1038/nature06116
Garcia-Romo GS, Caielli S, Vega B et al (2011) Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 3(73):73ra20. https://doi.org/10.1126/scitranslmed.3001201
Gestermann N, Di Domizio J, Lande R et al (2018) Netting neutrophils activate autoreactive B cells in lupus. J Immunol 200(10):3364–3371. https://doi.org/10.4049/jimmunol.1700778
Tillack K, Breiden P, Martin R et al (2012) T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J Immunol 188(7):3150–3159. https://doi.org/10.4049/jimmunol.1103414
Schauer C, Janko C, Munoz LE et al (2014) Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med 20(5):511–517. https://doi.org/10.1038/nm.3547
Crow MK, Olferiev M, Kirou KA (2019) Type I Interferons in Autoimmune Disease. Annu Rev Pathol 14:369–393. https://doi.org/10.1146/annurev-pathol-020117-043952
Deng Y, Tsao BP (2014) Advances in lupus genetics and epigenetics. Curr Opin Rheumatol 26(5):482–492. https://doi.org/10.1097/BOR.0000000000000086
Brandt L, Hedberg H (1969) Impaired phagocytosis by peripheral blood granulocytes in systemic lupus erythematosus. Scand J Haematol 6(5):348–353. https://doi.org/10.1111/j.1600-0609.1969.tb02420.x
Donnelly S, Roake W, Brown S et al (2006) Impaired recognition of apoptotic neutrophils by the C1q/calreticulin and CD91 pathway in systemic lupus erythematosus. Arthritis Rheum 54(5):1543–1556. https://doi.org/10.1002/art.21783
Lood C, Blanco LP, Purmalek MM et al (2016) Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med 22(2):146–153. https://doi.org/10.1038/nm.4027
Lindau D, Mussard J, Rabsteyn A et al (2014) TLR9 independent interferon alpha production by neutrophils on NETosis in response to circulating chromatin, a key lupus autoantigen. Ann Rheum Dis 73(12):2199–2207. https://doi.org/10.1136/annrheumdis-2012-203041
Palanichamy A, Bauer JW, Yalavarthi S et al (2014) Neutrophil-mediated IFN activation in the bone marrow alters B cell development in human and murine systemic lupus erythematosus. J Immunol 192(3):906–918. https://doi.org/10.4049/jimmunol.1302112
Rahman S, Sagar D, Hanna RN et al (2019) Low-density granulocytes activate T cells and demonstrate a non-suppressive role in systemic lupus erythematosus. Ann Rheum Dis 78(7):957–966. https://doi.org/10.1136/annrheumdis-2018-214620
van der Linden M, van den Hoogen LL, Westerlaken GHA et al (2018) Neutrophil extracellular trap release is associated with antinuclear antibodies in systemic lupus erythematosus and anti-phospholipid syndrome. Rheumatology (Oxford) 57(7):1228–1234. https://doi.org/10.1093/rheumatology/key067
Midgley A, Beresford MW (2016) Increased expression of low density granulocytes in juvenile-onset systemic lupus erythematosus patients correlates with disease activity. Lupus 25(4):407–411. https://doi.org/10.1177/0961203315608959
Carlucci PM, Purmalek MM, Dey AK et al (2018) Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. JCI Insight 3 (8). https://doi.org/10.1172/jci.insight.99276
Sagiv JY, Michaeli J, Assi S et al (2015) Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep 10(4):562–573. https://doi.org/10.1016/j.celrep.2014.12.039
Wu H, Zhen Y, Ma Z et al (2016) Arginase-1-dependent promotion of TH17 differentiation and disease progression by MDSCs in systemic lupus erythematosus. Sci Transl Med 8(331):331ra340. https://doi.org/10.1126/scitranslmed.aae0482
Marini O, Costa S, Bevilacqua D et al (2017) Mature CD10(+) and immature CD10(-) neutrophils present in G-CSF-treated donors display opposite effects on T cells. Blood 129(10):1343–1356. https://doi.org/10.1182/blood-2016-04-713206
Mistry P, Nakabo S, O’Neil L et al (2019) Transcriptomic, epigenetic, and functional analyses implicate neutrophil diversity in the pathogenesis of systemic lupus erythematosus. Proc Natl Acad Sci U S A 116(50):25222–25228. https://doi.org/10.1073/pnas.1908576116
Hakkim A, Furnrohr BG, Amann K et al (2010) Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A 107(21):9813–9818. https://doi.org/10.1073/pnas.0909927107
Villanueva E, Yalavarthi S, Berthier CC et al (2011) Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 187(1):538–552. https://doi.org/10.4049/jimmunol.1100450
Wang H, Li T, Chen S et al (2015) Neutrophil extracellular trap mitochondrial DNA and its autoantibody in systemic lupus erythematosus and a proof-of-concept trial of metformin. Arthritis Rheumatol 67(12):3190–3200. https://doi.org/10.1002/art.39296
Lande R, Ganguly D, Facchinetti V et al (2011) Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med 3(73):73ra19. https://doi.org/10.1126/scitranslmed.3001180
Bruschi M, Bonanni A, Petretto A et al (2020) Neutrophil extracellular traps profiles in patients with incident systemic lupus erythematosus and lupus nephritis. J Rheumatol 47(3):377–386. https://doi.org/10.3899/jrheum.181232
Odqvist L, Jevnikar Z, Riise R et al (2019) Genetic variations in A20 DUB domain provide a genetic link to citrullination and neutrophil extracellular traps in systemic lupus erythematosus. Ann Rheum Dis 78(10):1363–1370. https://doi.org/10.1136/annrheumdis-2019-215434
Liu E, Perl A (2019) Pathogenesis and treatment of autoimmune rheumatic diseases. Curr Opin Rheumatol 31(3):307–315. https://doi.org/10.1097/BOR.0000000000000594
Chang HH, Dwivedi N, Nicholas AP et al (2015) The W620 polymorphism in PTPN22 disrupts its interaction with peptidylarginine deiminase type 4 and enhances citrullination and NETosis. Arthritis Rheumatol 67(9):2323–2334. https://doi.org/10.1002/art.39215
Li D, Matta B, Song S et al (2020) IRF5 genetic risk variants drive myeloid-specific IRF5 hyperactivation and presymptomatic SLE. JCI Insight 5 (2). https://doi.org/10.1172/jci.insight.124020
Blazkova J, Gupta S, Liu Y et al (2017) Multicenter systems analysis of human blood reveals immature neutrophils in males and during pregnancy. J Immunol 198(6):2479–2488. https://doi.org/10.4049/jimmunol.1601855
Zhao Y, Wei W, Liu ML (2020) Extracellular vesicles and lupus nephritis - new insights into pathophysiology and clinical implications. J Autoimmun:102540. https://doi.org/10.1016/j.jaut.2020.102540
Gehrke N, Mertens C, Zillinger T et al (2013) Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity 39(3):482–495. https://doi.org/10.1016/j.immuni.2013.08.004
Goel RR, Nakabo S, Dizon BLP et al (2020) Lupus-like autoimmunity and increased interferon response in patients with STAT3-deficient hyper-IgE syndrome. J Allergy Clin Immunol. https://doi.org/10.1016/j.jaci.2020.07.024
Frangou E, Chrysanthopoulou A, Mitsios A et al (2019) REDD1/autophagy pathway promotes thromboinflammation and fibrosis in human systemic lupus erythematosus (SLE) through NETs decorated with tissue factor (TF) and interleukin-17A (IL-17A). Ann Rheum Dis 78(2):238–248. https://doi.org/10.1136/annrheumdis-2018-213181
Pieterse E, Rother N, Garsen M et al (2017) Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. Arterioscler Thromb Vasc Biol 37(7):1371–1379. https://doi.org/10.1161/ATVBAHA.117.309002
Carmona-Rivera C, Zhao W, Yalavarthi S et al (2015) Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis 74(7):1417–1424. https://doi.org/10.1136/annrheumdis-2013-204837
Shen M, Zeng X, Tian X et al (2010) Diffuse alveolar hemorrhage in systemic lupus erythematosus: a retrospective study in China. Lupus 19(11):1326–1330. https://doi.org/10.1177/0961203310373106
Jarrot PA, Tellier E, Plantureux L et al (2019) Neutrophil extracellular traps are associated with the pathogenesis of diffuse alveolar hemorrhage in murine lupus. J Autoimmun 100:120–130. https://doi.org/10.1016/j.jaut.2019.03.009
Pillinger MH, Abramson SB (1995) The neutrophil in rheumatoid arthritis. Rheum Dis Clin North Am 21(3):691–714
Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A et al (2013) NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med 5(178):178ra140. https://doi.org/10.1126/scitranslmed.3005580
Wright HL, Makki FA, Moots RJ et al (2017) Low-density granulocytes: functionally distinct, immature neutrophils in rheumatoid arthritis with altered properties and defective TNF signalling. J Leukoc Biol 101(2):599–611. https://doi.org/10.1189/jlb.5A0116-022R
Too CL, Murad S, Dhaliwal JS et al (2012) Polymorphisms in peptidylarginine deiminase associate with rheumatoid arthritis in diverse Asian populations: evidence from MyEIRA study and meta-analysis. Arthritis Res Ther 14(6):R250. https://doi.org/10.1186/ar4093
Sohn DH, Rhodes C, Onuma K et al (2015) Local Joint inflammation and histone citrullination in a murine model of the transition from preclinical autoimmunity to inflammatory arthritis. Arthritis Rheumatol 67(11):2877–2887. https://doi.org/10.1002/art.39283
Carmona-Rivera C, Carlucci PM, Moore E et al (2017) Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci Immunol 2 (10). https://doi.org/10.1126/sciimmunol.aag3358
Nakabo S, Ohmura K, Akizuki S et al (2020) Activated neutrophil carbamylates albumin via the release of myeloperoxidase and reactive oxygen species regardless of NETosis. Mod Rheumatol 30(2):345–349. https://doi.org/10.1080/14397595.2019.1583819
Papadaki G, Kambas K, Choulaki C et al (2016) Neutrophil extracellular traps exacerbate Th1-mediated autoimmune responses in rheumatoid arthritis by promoting DC maturation. Eur J Immunol 46(11):2542–2554. https://doi.org/10.1002/eji.201646542
Carmona-Rivera C, Carlucci PM, Goel RR et al (2020) Neutrophil extracellular traps mediate articular cartilage damage and enhance cartilage component immunogenicity in rheumatoid arthritis. JCI Insight 5 (13). https://doi.org/10.1172/jci.insight.139388
Ribon M, Seninet S, Mussard J et al (2019) Neutrophil extracellular traps exert both pro- and anti-inflammatory actions in rheumatoid arthritis that are modulated by C1q and LL-37. J Autoimmun 98:122–131. https://doi.org/10.1016/j.jaut.2019.01.003
Wu XY, Li KT, Yang HX et al (2020) Complement C1q synergizes with PTX3 in promoting NLRP3 inflammasome over-activation and pyroptosis in rheumatoid arthritis. J Autoimmun 106:102336. https://doi.org/10.1016/j.jaut.2019.102336
Sule G, Kelley WJ, Gockman K et al (2020) Increased adhesive potential of antiphospholipid syndrome neutrophils mediated by beta2 integrin Mac-1. Arthritis Rheumatol 72(1):114–124. https://doi.org/10.1002/art.41057
Knight JS, Meng H, Coit P et al (2017) Activated signature of antiphospholipid syndrome neutrophils reveals potential therapeutic target. JCI Insight 2 (18). https://doi.org/10.1172/jci.insight.93897
van den Hoogen LL, Fritsch-Stork RD, van Roon JA et al (2016) Low-density granulocytes are increased in antiphospholipid syndrome and are associated with anti-beta2-glycoprotein I antibodies: comment on the article by Yalavarthi, et al. Arthritis Rheumatol 68(5):1320–1321. https://doi.org/10.1002/art.39576
Yalavarthi S, Gould TJ, Rao AN et al (2015) Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol 67(11):2990–3003. https://doi.org/10.1002/art.39247
Zuo Y, Yalavarthi S, Gockman K et al (2020) Anti-NET antibodies and impaired NET degradation in antiphospholipid syndrome. Arthritis Rheumatol. https://doi.org/10.1002/art.41460
van den Hoogen LL, van der Linden M, Meyaard L et al (2020) Neutrophil extracellular traps and low-density granulocytes are associated with the interferon signature in systemic lupus erythematosus, but not in antiphospholipid syndrome. Ann Rheum Dis 79(10):e135. https://doi.org/10.1136/annrheumdis-2019-215781
Girardi G, Berman J, Redecha P et al (2003) Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest 112(11):1644–1654. https://doi.org/10.1172/JCI18817
Redecha P, Tilley R, Tencati M et al (2007) Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood 110(7):2423–2431. https://doi.org/10.1182/blood-2007-01-070631
Marder W, Knight JS, Kaplan MJ et al (2016) Placental histology and neutrophil extracellular traps in lupus and pre-eclampsia pregnancies. Lupus Sci Med 3(1):e000134. https://doi.org/10.1136/lupus-2015-000134
Zha C, Zhang W, Gao F et al (2018) Anti-beta2GPI/beta2GPI induces neutrophil extracellular traps formation to promote thrombogenesis via the TLR4/MyD88/MAPKs axis activation. Neuropharmacology 138:140–150. https://doi.org/10.1016/j.neuropharm.2018.06.001
Meng H, Yalavarthi S, Kanthi Y et al (2017) In vivo role of neutrophil extracellular traps in antiphospholipid antibody-mediated venous thrombosis. Arthritis Rheumatol 69(3):655–667. https://doi.org/10.1002/art.39938
Ali RA, Gandhi AA, Meng H et al (2019) Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat Commun 10(1):1916. https://doi.org/10.1038/s41467-019-09801-x
Kitching AR, Anders HJ, Basu N et al (2020) ANCA-associated vasculitis Nat Rev Dis Primers 6(1):71. https://doi.org/10.1038/s41572-020-0204-y
Kessenbrock K, Krumbholz M, Schonermarck U et al (2009) Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 15(6):623–625. https://doi.org/10.1038/nm.1959
Falk RJ, Terrell RS, Charles LA et al (1990) Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci U S A 87(11):4115–4119. https://doi.org/10.1073/pnas.87.11.4115
Schreiber A, Rousselle A, Becker JU et al (2017) Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc Natl Acad Sci U S A 114(45):E9618–E9625. https://doi.org/10.1073/pnas.1708247114
Carmona-Rivera C, Purmalek MM, Moore E et al (2017) A role for muscarinic receptors in neutrophil extracellular trap formation and levamisole-induced autoimmunity. JCI Insight 2(3):e89780. https://doi.org/10.1172/jci.insight.89780
Lood C, Hughes GC (2017) Neutrophil extracellular traps as a potential source of autoantigen in cocaine-associated autoimmunity. Rheumatology (Oxford) 56(4):638–643. https://doi.org/10.1093/rheumatology/kew256
Kraaij T, Kamerling SWA, van Dam LS et al (2018) Excessive neutrophil extracellular trap formation in ANCA-associated vasculitis is independent of ANCA. Kidney Int 94(1):139–149. https://doi.org/10.1016/j.kint.2018.01.013
van Dam LS, Kraaij T, Kamerling SWA et al (2019) Intrinsically distinct role of neutrophil extracellular trap formation in antineutrophil cytoplasmic antibody-associated vasculitis compared to systemic lupus erythematosus. Arthritis Rheumatol 71(12):2047–2058. https://doi.org/10.1002/art.41047
Grayson PC, Carmona-Rivera C, Xu L et al (2015) Neutrophil-related gene expression and low-density granulocytes associated with disease activity and response to treatment in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol 67(7):1922–1932. https://doi.org/10.1002/art.39153
Cheadle C, Berger AE, Andrade F et al (2010) Transcription of proteinase 3 and related myelopoiesis genes in peripheral blood mononuclear cells of patients with active Wegener’s granulomatosis. Arthritis Rheum 62(6):1744–1754. https://doi.org/10.1002/art.27398
Lyons PA, McKinney EF, Rayner TF et al (2010) Novel expression signatures identified by transcriptional analysis of separated leucocyte subsets in systemic lupus erythematosus and vasculitis. Ann Rheum Dis 69(6):1208–1213. https://doi.org/10.1136/ard.2009.108043
Nakazawa D, Shida H, Tomaru U et al (2014) Enhanced formation and disordered regulation of NETs in myeloperoxidase-ANCA-associated microscopic polyangiitis. J Am Soc Nephrol 25(5):990–997. https://doi.org/10.1681/ASN.2013060606
Sangaletti S, Tripodo C, Chiodoni C et al (2012) Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood 120(15):3007–3018. https://doi.org/10.1182/blood-2012-03-416156
Kambas K, Chrysanthopoulou A, Vassilopoulos D et al (2014) Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann Rheum Dis 73(10):1854–1863. https://doi.org/10.1136/annrheumdis-2013-203430
Huang YM, Wang H, Wang C et al (2015) Promotion of hypercoagulability in antineutrophil cytoplasmic antibody-associated vasculitis by C5a-induced tissue factor-expressing microparticles and neutrophil extracellular traps. Arthritis Rheumatol 67(10):2780–2790. https://doi.org/10.1002/art.39239
Nakazawa D, Tomaru U, Yamamoto C et al (2012) Abundant neutrophil extracellular traps in thrombus of patient with microscopic polyangiitis. Front Immunol 3:333. https://doi.org/10.3389/fimmu.2012.00333
O’Sullivan KM, Lo CY, Summers SA et al (2015) Renal participation of myeloperoxidase in antineutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis. Kidney Int 88(5):1030–1046. https://doi.org/10.1038/ki.2015.202
Yoshida M, Yamada M, Sudo Y et al (2016) Myeloperoxidase anti-neutrophil cytoplasmic antibody affinity is associated with the formation of neutrophil extracellular traps in the kidney and vasculitis activity in myeloperoxidase anti-neutrophil cytoplasmic antibody-associated microscopic polyangiitis. Nephrology (Carlton) 21(7):624–629. https://doi.org/10.1111/nep.12736
Selva-O’Callaghan A, Pinal-Fernandez I, Trallero-Araguas E et al (2018) Classification and management of adult inflammatory myopathies. Lancet Neurol 17(9):816–828. https://doi.org/10.1016/S1474-4422(18)30254-0
Zhang S, Shu X, Tian X et al (2014) Enhanced formation and impaired degradation of neutrophil extracellular traps in dermatomyositis and polymyositis: a potential contributor to interstitial lung disease complications. Clin Exp Immunol 177(1):134–141. https://doi.org/10.1111/cei.12319
Seto N, Torres-Ruiz JJ, Carmona-Rivera C et al (2020) Neutrophil dysregulation is pathogenic in idiopathic inflammatory myopathies. JCI Insight 5 (3). https://doi.org/10.1172/jci.insight.134189
Zhang S, Shen H, Shu X et al (2017) Abnormally increased low-density granulocytes in peripheral blood mononuclear cells are associated with interstitial lung disease in dermatomyositis. Mod Rheumatol 27(1):122–129. https://doi.org/10.1080/14397595.2016.1179861
Masters SL, Simon A, Aksentijevich I et al (2009) Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*). Annu Rev Immunol 27:621–668. https://doi.org/10.1146/annurev.immunol.25.022106.141627
Frizinsky S, Haj-Yahia S, Machnes Maayan D et al (2019) The innate immune perspective of autoimmune and autoinflammatory conditions. Rheumatology (Oxford) 58(Suppl 6):vi1–vi8. https://doi.org/10.1093/rheumatology/kez387
Mistry P, Carmona-Rivera C, Ombrello AK et al (2018) Dysregulated neutrophil responses and neutrophil extracellular trap formation and degradation in PAPA syndrome. Ann Rheum Dis 77(12):1825–1833. https://doi.org/10.1136/annrheumdis-2018-213746
Dierselhuis MP, Frenkel J, Wulffraat NM et al (2005) Anakinra for flares of pyogenic arthritis in PAPA syndrome. Rheumatology (Oxford) 44(3):406–408. https://doi.org/10.1093/rheumatology/keh479
Li S, Zheng S, Tang S et al (2020) Autoinflammatory pathogenesis and targeted therapy for adult-onset Still’s disease. Clin Rev Allergy Immunol 58(1):71–81. https://doi.org/10.1007/s12016-019-08747-8
Torres-Ruiz J, Carrillo-Vazquez DA, Tapia-Rodriguez M et al (2019) The role of low density granulocytes and NETosis in the pathogenesis of adult-onset Still’s disease. Clin Exp Rheumatol 37(Suppl 121 (6)):74–82
Hu Q, Shi H, Zeng T et al (2019) Increased neutrophil extracellular traps activate NLRP3 and inflammatory macrophages in adult-onset Still’s disease. Arthritis Res Ther 21(1):9. https://doi.org/10.1186/s13075-018-1800-z
Ramanathan K, Glaser A, Lythgoe H et al (2018) Neutrophil activation signature in juvenile idiopathic arthritis indicates the presence of low-density granulocytes. Rheumatology (Oxford) 57(3):488–498. https://doi.org/10.1093/rheumatology/kex441
Prete F, Catucci M, Labrada M et al (2013) Wiskott-Aldrich syndrome protein-mediated actin dynamics control type-I interferon production in plasmacytoid dendritic cells. J Exp Med 210(2):355–374. https://doi.org/10.1084/jem.20120363
Cervantes-Luevano KE, Caronni N, Castiello MC et al (2018) Neutrophils drive type I interferon production and autoantibodies in patients with Wiskott-Aldrich syndrome. J Allergy Clin Immunol 142 (5):1605–1617 e1604. https://doi.org/10.1016/j.jaci.2017.11.063
Lima AL, Karl I, Giner T et al (2016) Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol 174(3):514–521. https://doi.org/10.1111/bjd.14214
Byrd AS, Carmona-Rivera C, O’Neil LJ et al (2019) Neutrophil extracellular traps, B cells, and type I interferons contribute to immune dysregulation in hidradenitis suppurativa. Sci Transl Med 11(508):eaav5908. https://doi.org/10.1126/scitranslmed.aav5908
Becatti M, Emmi G, Silvestri E et al (2016) Neutrophil activation promotes fibrinogen oxidation and thrombus formation in Behcet disease. Circulation 133(3):302–311. https://doi.org/10.1161/CIRCULATIONAHA.115.017738
Safi R, Kallas R, Bardawil T et al (2018) Neutrophils contribute to vasculitis by increased release of neutrophil extracellular traps in Behcet’s disease. J Dermatol Sci 92(2):143–150. https://doi.org/10.1016/j.jdermsci.2018.08.010
Le Joncour A, Martos R, Loyau S et al (2019) Critical role of neutrophil extracellular traps (NETs) in patients with Behcet’s disease. Ann Rheum Dis 78(9):1274–1282. https://doi.org/10.1136/annrheumdis-2018-214335
Zhou Q, Yang D, Ombrello AK et al (2014) Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med 370(10):911–920. https://doi.org/10.1056/NEJMoa1307361
Navon Elkan P, Pierce SB, Segel R et al (2014) Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N Engl J Med 370(10):921–931. https://doi.org/10.1056/NEJMoa1307362
Carmona-Rivera C, Khaznadar SS, Shwin KW et al (2019) Deficiency of adenosine deaminase 2 triggers adenosine-mediated NETosis and TNF production in patients with DADA2. Blood 134(4):395–406. https://doi.org/10.1182/blood.2018892752
Lopez P, Rodriguez-Carrio J, Martinez-Zapico A et al (2020) Low-density granulocytes and monocytes as biomarkers of cardiovascular risk in systemic lupus erythematosus. Rheumatology (Oxford) 59(7):1752–1764. https://doi.org/10.1093/rheumatology/keaa016
Jourde-Chiche N, Whalen E, Gondouin B et al (2017) Modular transcriptional repertoire analyses identify a blood neutrophil signature as a candidate biomarker for lupus nephritis. Rheumatology (Oxford) 56(3):477–487. https://doi.org/10.1093/rheumatology/kew439
Wright HL, Cox T, Moots RJ et al (2017) Neutrophil biomarkers predict response to therapy with tumor necrosis factor inhibitors in rheumatoid arthritis. J Leukoc Biol 101(3):785–795. https://doi.org/10.1189/jlb.5A0616-258R
Ghang B, Kwon O, Hong S et al (2017) Neutrophil-to-lymphocyte ratio is a reliable marker of treatment response in rheumatoid arthritis patients during tocilizumab therapy. Mod Rheumatol 27(3):405–410. https://doi.org/10.1080/14397595.2016.1214340
Moore S, Juo HH, Nielsen CT et al (2019) Role of neutrophil extracellular traps regarding patients at risk of increased disease activity and cardiovascular comorbidity in systemic lupus erythematosus. J Rheumatol. https://doi.org/10.3899/jrheum.190875
Leffler J, Gullstrand B, Jonsen A et al (2013) Degradation of neutrophil extracellular traps co-varies with disease activity in patients with systemic lupus erythematosus. Arthritis Res Ther 15(4):R84. https://doi.org/10.1186/ar4264
Liu Y, Carmona-Rivera C, Moore E et al (2018) Myeloid-specific deletion of peptidylarginine deiminase 4 mitigates atherosclerosis. Front Immunol 9:1680. https://doi.org/10.3389/fimmu.2018.01680
Knight JS, Luo W, O’Dell AA et al (2014) Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ Res 114(6):947–956. https://doi.org/10.1161/CIRCRESAHA.114.303312
Warnatsch A, Ioannou M, Wang Q et al (2015) Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 349(6245):316–320. https://doi.org/10.1126/science.aaa8064
Perez-Sanchez C, Ruiz-Limon P, Aguirre MA et al (2017) Diagnostic potential of NETosis-derived products for disease activity, atherosclerosis and therapeutic effectiveness in Rheumatoid Arthritis patients. J Autoimmun 82:31–40. https://doi.org/10.1016/j.jaut.2017.04.007
Moots RJ, Sebba A, Rigby W et al (2017) Effect of tocilizumab on neutrophils in adult patients with rheumatoid arthritis: pooled analysis of data from phase 3 and 4 clinical trials. Rheumatology (Oxford) 56(4):541–549. https://doi.org/10.1093/rheumatology/kew370
Taylor PC, Peters AM, Paleolog E et al (2000) Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor alpha blockade in patients with rheumatoid arthritis. Arthritis Rheum 43(1):38–47. https://doi.org/10.1002/1529-0131(200001)43:1<38::AID-ANR6>3.0.CO;2-L
Mitchell TS, Moots RJ, Wright HL (2017) Janus kinase inhibitors prevent migration of rheumatoid arthritis neutrophils towards interleukin-8, but do not inhibit priming of the respiratory burst or reactive oxygen species production. Clin Exp Immunol 189(2):250–258. https://doi.org/10.1111/cei.12970
Furumoto Y, Smith CK, Blanco L et al (2017) Tofacitinib ameliorates murine lupus and its associated vascular dysfunction. Arthritis Rheumatol 69(1):148–160. https://doi.org/10.1002/art.39818
Smith CK, Vivekanandan-Giri A, Tang C et al (2014) Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol 66(9):2532–2544. https://doi.org/10.1002/art.38703
Willis VC, Banda NK, Cordova KN et al (2017) Protein arginine deiminase 4 inhibition is sufficient for the amelioration of collagen-induced arthritis. Clin Exp Immunol 188(2):263–274. https://doi.org/10.1111/cei.12932
Willis VC, Gizinski AM, Banda NK et al (2011) N-Alpha-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J Immunol 186(7):4396–4404. https://doi.org/10.4049/jimmunol.1001620
Knight JS, Zhao W, Luo W et al (2013) Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J Clin Invest 123(7):2981–2993. https://doi.org/10.1172/JCI67390
Knight JS, Subramanian V, O’Dell AA et al (2015) Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann Rheum Dis 74(12):2199–2206. https://doi.org/10.1136/annrheumdis-2014-205365
Kusunoki Y, Nakazawa D, Shida H et al (2016) Peptidylarginine deiminase inhibitor suppresses neutrophil extracellular trap formation and MPO-ANCA production. Front Immunol 7:227. https://doi.org/10.3389/fimmu.2016.00227
Kumar SV, Kulkarni OP, Mulay SR et al (2015) Neutrophil extracellular trap-related extracellular histones cause vascular necrosis in severe GN. J Am Soc Nephrol 26(10):2399–2413. https://doi.org/10.1681/ASN.2014070673
Erpenbeck L, Chowdhury CS, Zsengeller ZK et al (2016) PAD4 deficiency decreases inflammation and susceptibility to pregnancy loss in a mouse model. Biol Reprod 95(6):132. https://doi.org/10.1095/biolreprod.116.140293
Mizugishi K, Yamashita K (2017) Neutrophil extracellular traps are critical for pregnancy loss in sphingosine kinase-deficient mice on 129Sv/C57BL/6 background. FASEB J 31(12):5577–5591. https://doi.org/10.1096/fj.201700399RR
Prasad M, Hermann J, Gabriel SE et al (2015) Cardiorheumatology: cardiac involvement in systemic rheumatic disease. Nat Rev Cardiol 12(3):168–176. https://doi.org/10.1038/nrcardio.2014.206
Liu Y, Kaplan MJ (2018) Cardiovascular disease in systemic lupus erythematosus: an update. Curr Opin Rheumatol 30(5):441–448. https://doi.org/10.1097/BOR.0000000000000528
Liu Y, Lightfoot YL, Seto N et al (2018) Peptidylarginine deiminases 2 and 4 modulate innate and adaptive immune responses in TLR-7-dependent lupus. JCI Insight 3 (23). https://doi.org/10.1172/jci.insight.124729
Hanata N, Shoda H, Hatano H et al (2020) Peptidylarginine deiminase 4 promotes the renal infiltration of neutrophils and exacerbates the TLR7 agonist-induced lupus mice. Front Immunol 11:1095. https://doi.org/10.3389/fimmu.2020.01095
Kienhofer D, Hahn J, Stoof J et al (2017) Experimental lupus is aggravated in mouse strains with impaired induction of neutrophil extracellular traps. JCI Insight 2 (10). https://doi.org/10.1172/jci.insight.92920
Gordon RA, Herter JM, Rosetti F et al (2017) Lupus and proliferative nephritis are PAD4 independent in murine models. JCI Insight 2 (10). https://doi.org/10.1172/jci.insight.92926
Rohrbach AS, Hemmers S, Arandjelovic S et al (2012) PAD4 is not essential for disease in the K/BxN murine autoantibody-mediated model of arthritis. Arthritis Res Ther 14(3):R104. https://doi.org/10.1186/ar3829
Shelef MA, Sokolove J, Lahey LJ et al (2014) Peptidylarginine deiminase 4 contributes to tumor necrosis factor alpha-induced inflammatory arthritis. Arthritis Rheumatol 66(6):1482–1491. https://doi.org/10.1002/art.38393
Seri Y, Shoda H, Suzuki A et al (2015) Peptidylarginine deiminase type 4 deficiency reduced arthritis severity in a glucose-6-phosphate isomerase-induced arthritis model. Sci Rep 5:13041. https://doi.org/10.1038/srep13041
Chirivi RGS, van Rosmalen JWG, van der Linden M et al (2020) Therapeutic ACPA inhibits NET formation: a potential therapy for neutrophil-mediated inflammatory diseases. Cell Mol Immunol. https://doi.org/10.1038/s41423-020-0381-3
Blanco LP, Pedersen HL, Wang X et al (2020) Improved mitochondrial metabolism and reduced inflammation following attenuation of murine lupus with coenzyme Q10 analog idebenone. Arthritis Rheumatol 72(3):454–464. https://doi.org/10.1002/art.41128
Fortner KA, Blanco LP, Buskiewicz I et al (2020) Targeting mitochondrial oxidative stress with MitoQ reduces NET formation and kidney disease in lupus-prone MRL-lpr mice. Lupus Sci Med 7(1):e000387. https://doi.org/10.1136/lupus-2020-000387
Funding
Yudong Liu was supported by funds from the National Natural Science Foundation of China, grant no. 81971521, and Peking University People’s Hospital Research and Development Fund, grant no. RDY2019-14. Mariana J. Kaplan was supported by the Intramural Research Program at NIAMS/NIH, ZIAAR041199.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Kaplan, M.J. Neutrophils in the Pathogenesis of Rheumatic Diseases: Fueling the Fire. Clinic Rev Allerg Immunol 60, 1–16 (2021). https://doi.org/10.1007/s12016-020-08816-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-020-08816-3