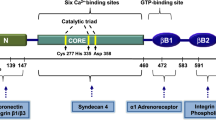
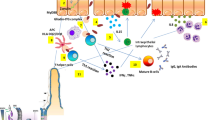
Abstract
Celiac disease is a common inflammatory disorder with a prevalence of 1–2 % in which a distinct dietary wheat, rye, and barley component, gluten, induces small-bowel mucosal villous atrophy, crypt hyperplasia, and inflammation. The small-bowel mucosal damage can be reversed by a strict lifelong gluten-free diet, which is currently the only effective treatment for the condition. A key player in the pathogenetic process leading to the enteropathy is played by a protein called transglutaminase 2 (TG2), which is able to enzymatically modify gluten-derived gliadin peptides. The TG2-catalyzed deamidation of the gliadin peptides results in their increased binding affinity to the disease-predisposing human leukocyte antigen (HLA) DQ2 and DQ8 molecules, thus enabling a strong immune response to be launched. Blocking the enzymatic activity of TG2 has thus been suggested as a suitable novel pharmacological approach to treat celiac disease. By virtue of its transamidation capacity, TG2 is also able to cross-link gliadin peptides to itself, this resulting in the generation of TG2-gliadin peptide complexes whose presence might provide an explanation for the generation of the TG2 autoantibodies characteristic of celiac disease. Due to their excellent specificity for the disorder, the TG2-targeted autoantibodies are widely used in the diagnostics as a first-line test to select patients for gastrointestinal endoscopy. More recently, it has come to be appreciated that these autoantibodies and also the TG2-specific B cells might play an active role in the disease pathogenesis. In this review, we assess the role of TG2, TG2-specific B cells, and autoantibodies in celiac disease.


Similar content being viewed by others
References
Lorand L, Graham R (2003) Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 4:140–156
Griffin M, Casadio R, Bergamini C (2002) Transglutaminases: nature’s biological glues. Biochem J 368:377–396
Sarkar NK, Clarke DD, Waelsch H (1957) An enzymically catalyzed incorporation of amines into proteins. Biochim Biophys Acta 25:451–452
Folk JEFJ (1977) The epsilon-(gamma-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Adv Protein Chem 31:1–133
Gentile V, Davies P, Baldini A (1994) The human tissue transglutaminase gene maps on chromosome 20q12 by in situ fluorescence hybridization. Genomics 20:295–297
Liu S, Cerione R, Clardy J (2002) Structural basis for the guanine nucleotide-binding activity of tissue transglutaminase and its regulation of transamidation activity. Proc Natl Acad Sci U S A 99:2743–2747
Pinkas DM, Strop P, Brunger AT, Khosla C (2007) Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol 5:2788–2796
Lai T, Greenberg CS (2013) TGM2 and implications for human disease: role of alternative splicing. Front Biosci 18:504–519
Nurminskaya MV, Belkin AM (2012) Cellular functions of tissue transglutaminase. Int Rev Cell Mol Biol 294:1–97
Davies PJ, Murtaugh MP, Moore WT Jr, Johnson GS, Lucas D (1985) Retinoic acid-induced expression of tissue transglutaminase in human promyelocytic leukemia (HL-60) cells. J Biol Chem 260:5166–5174
Park D, Choi SS, Ha K (2010) Transglutaminase 2: a multi-functional protein in multiple subcellular compartments. Amino Acids 39:619–631
Zemskov EA, Mikhailenko I, Hsia R, Zaritskaya L, Belkin AM (2011) Unconventional secretion of tissue transglutaminase involves phospholipid-dependent delivery into recycling endosomes. FEBS J 278:96–96
Achyuthan KE, Greenberg CS (1987) Identification of a guanosine triphosphate-binding site on guinea pig liver transglutaminase. Role of GTP and calcium ions in modulating activity. J Biol Chem 262:1901–1906
Stamnaes J, Pinkas DM, Fleckenstein B, Khosla C, Sollid LM (2010) Redox regulation of transglutaminase 2 activity. J Biol Chem 285:25402–25409
Siegel M, Strnad P, Watts RE, Choi K, Jabri B, Omary MB, Khosla C (2008) Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury. PLoS One 3:e1861
Jin X, Stamnaes J, Klock C, DiRaimondo TR, Sollid LM, Khosla C (2011) Activation of extracellular transglutaminase 2 by thioredoxin. J Biol Chem 286:37866–37873
Lai T, Hausladen A, Slaughter T, Eu J, Stamler J, Greenberg C (2001) Calcium regulates S-nitrosylation, denitrosylation, and activity of tissue transglutaminase. Biotechnology (N Y) 40:4904–4910
Mishra S, Murphy L (2004) Tissue transglutaminase has intrinsic kinase activity—identification of transglutaminase 2 as an insulin-like growth factor-binding protein-3 kinase. J Biol Chem 279:23863–23868
Mishra S, Melino G, Murphy LJ (2007) Transglutaminase 2 kinase activity facilitates protein kinase A-induced phosphorylation of retinoblastoma protein. J Biol Chem 282:18108–18115
Wang Z, Griffin M (2012) TG2, a novel extracellular protein with multiple functions. Amino Acids 42:939–949
Esposito C, Caputo I (2005) Mammalian transglutaminases—identification of substrates as a key to physiological function and physiopathological relevance. FEBS J 272:615–631
Kanchan K, Fuxreiter M, Fésüs L (2015) Physiological, pathological, and structural implications of non-enzymatic protein–protein interactions of the multifunctional human transglutaminase 2. Cell Mol Life Sci 72:3009–3035
Fleckenstein B, Molberg Y, Qiao S, Schmid D, von der Mullbe F, Elgstoen K, Jung G, Sollid L (2002) Gliadin T cell epitope selection by tissue transglutaminase in celiac disease—role of enzyme specificity and pH influence on the transamidation versus deamidation reactions. J Biol Chem 277:34109–34116
Nakaoka H, Perez D, Baek K, Das T, Husain A, Misono K, Im M, Graham R (1994) G(h)—a Gtp-binding protein with transglutaminase activity and receptor signaling function. Science 264:1593–1596
Iismaa SE, Chung L, Wu M, Teller DC, Yee VC, Graham RM (1997) The core domain of the tissue transglutaminase Gh hydrolyzes GTP and ATP. Biochemistry 36:11655–1166 4
Hasegawa G, Suwa M, Ichikawa Y, Ohtsuka T, Kumagai S, Kikuchi M, Sato Y, Saito Y (2003) A novel function of tissue-type transglutaminase: protein disulphide isomerase. Biochem J 373:793–803
Mishra S, Saleh A, Espino PS, Davie JR, Murphy LJ (2006) Phosphorylation of histones by tissue transglutaminase. J Biol Chem 281:5532–5538
Akimov S, Krylov D, Fleischman L, Belkin A (2000) Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol 148:825–838
Akimov S, Belkin A (2001) Cell surface tissue transglutaminase is involved in adhesion and migration of monocytic cells on fibronectin. Blood 98:1567–1576
Iismaa SE, Mearns BM, Lorand L, Graham RM (2009) Transglutaminases and disease: lessons from genetically engineered mouse models and inherited disorders. Physiol Rev 89:991–1023
Sulkanen S, Halttunen T, Laurila K, Kolho K, Korponay-Szabó IR, Sarnesto A, Savilahti E, Collin P, Mäki M (1998) Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology 115:1322–1328
Chorzelski TP, Sulej J, Tchorzewska H, Jablonska S, Beutner EH, Kumar V (1983) IgA class endomysium antibodies in dermatitis herpetiformis and coeliac disease a. Ann N Y Acad Sci 420:325–334
Rashtak S, Ettore MW, Homburger HA, Murray JA (2008) Comparative usefulness of deamidated gliadin antibodies in the diagnosis of celiac disease. Clin Gastroenterol Hepatol 6:426–432
Mustalahti K, Catassi C, Reunanen A, Fabiani E, Heier M, McMillan S, Murray L, Metzger M, Gasparin M, Bravi E (2010) The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med 42:587–595
Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, Elitsur Y, Green PH, Guandalini S, Hill ID (2003) Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med 163:286–292
Lohi S, Mustalahti K, Kaukinen K, Laurila K, Collin P, Rissanen H, Lohi O, Bravi E, Gasparin M, Reunanen A (2007) Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther 26:1217–1225
Green PH, Lebwohl B, Greywoode R (2015) Celiac disease. J Allergy Clin Immunol 135:1099–1106
Mäki M, Mustalahti K, Kokkonen J, Kulmala P, Haapalahti M, Karttunen T, Ilonen J, Laurila K, Dahlbom I, Hansson T (2003) Prevalence of celiac disease among children in Finland. N Engl J Med 348:2517–2524
Vilppula A, Kaukinen K, Luostarinen L, Krekelä I, Patrikainen H, Valve R, Mäki M, Collin P (2009) Increasing prevalence and high incidence of celiac disease in elderly people: a population-based study. BMC Gastroenterol 9:1
Tack GJ, Verbeek WH, Schreurs MW, Mulder CJ (2010) The spectrum of celiac disease: epidemiology, clinical aspects and treatment. Nat Rev Gastroenterol Hepatol 7:204–213
Sollid LM (2004) Intraepithelial lymphocytes in celiac disease: license to kill revealed. Immunity 21:303–304
Akobeng AK, Ramanan AV, Buchan I, Heller RF (2006) Effect of breast feeding on risk of coeliac disease: a systematic review and meta-analysis of observational studies. Arch Dis Child 91:39–43
Silano M, Agostoni C, Guandalini S (2010) Effect of the timing of gluten introduction on the development of celiac disease. World J Gastroenterol 16:1939–1942
Vriezinga SL, Auricchio R, Bravi E, Castillejo G, Chmielewska A, Crespo Escobar P, Kolaček S, Koletzko S, Korponay-Szabo IR, Mummert E (2014) Randomized feeding intervention in infants at high risk for celiac disease. N Engl J Med 371:1304–1315
Silano M, Agostoni C, Sanz Y, Guandalini S (2016) Infant feeding and risk of developing celiac disease: a systematic review. BMJ Open 6:e009163–2015-009163
Mårild K, Kahrs CR, Tapia G, Stene LC, Størdal K (2015) Infections and risk of celiac disease in childhood: a prospective nationwide cohort study. Am J Gastroenterol 110:1475–1484
Stene LC, Honeyman MC, Hoffenberg EJ, Haas JE, Sokol RJ, Emery L, Taki I, Norris JM, Erlich HA, Eisenbarth GS (2006) Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol 101:2333–2340
Plot L, Amital H, Barzilai O, Ram M, Nicola B, Shoenfeld Y (2009) Infections may have a protective role in the etiopathogenesis of celiac disease. Ann N Y Acad Sci 1173:670–674
Kondrashova A, Mustalahti K, Kaukinen K, Viskari H, Volodicheva V, Haapala A, Ilonen J, Knip M, Mäki M, Hyöty H (2008) Lower economic status and inferior hygienic environment may protect against celiac disease. Ann Med 40:223–231
Dieli-Crimi R, Cénit MC, Núñez C (2015) The genetics of celiac disease: a comprehensive review of clinical implications. J Autoimmun 64:26–41
Hadithi M, Von Blomberg B, Mary E, Crusius JBA, Bloemena E, Kostense PJ, Meijer JW, Mulder CJ, Stehouwer CD (2007) Accuracy of serologic tests and HLA-DQ typing for diagnosing celiac disease. Ann Intern Med 147:294–302
Romanos J, Rosen A, Kumar V, Trynka G, Franke L, Szperl A, Gutierrez-Achury J, van Diemen CC, Kanninga R, SA J, Steck A, Eisenbarth G, van Heel DA, Cukrowska B, Bruno V, Mazzilli MC, Nunez C, Bilbao JR, Mearin ML, Barisani D, Rewers M, Norris JM, Ivarsson A, Boezen HM, Liu E, Wijmenga C, Prevent CD Group (2014) Improving coeliac disease risk prediction by testing non-HLA variants additional to HLA variants. Gut 63:415–422
Kivelä L, Kaukinen K, Lähdeaho M, Huhtala H, Ashorn M, Ruuska T, Hiltunen P, Visakorpi J, Mäki M, Kurppa K (2015) Presentation of celiac disease in Finnish children is no longer changing: a 50-year perspective. J Pediatr 167:1109–1115
Garampazzi A, Rapa A, Mura S, Capelli A, Valori A, Boldorini R, Oderda G (2007) Clinical pattern of celiac disease is still changing. J Pediatr Gastroenterol Nutr 45:611–614
Leffler DA, Green PH, Fasano A (2015) Extraintestinal manifestations of coeliac disease. Nat Rev Gastroenterol Hepatol 12:561–571
Reunala T, Salmi TT, Hervonen K (2015) Dermatitis herpetiformis: pathognomonic transglutaminase IgA deposits in the skin and excellent prognosis on a gluten-free diet. Acta Derm Venereol 95:917–922
Dieterich W, Laag E, Bruckner-Tuderman L, Reunala T, Kárpáti S, Zágoni T, Riecken EO, Schuppan D (1999) Antibodies to tissue transglutaminase as serologic markers in patients with dermatitis herpetiformis. J Investig Dermatol 113:133–136
Salmi TT, Hervonen K, Laurila K, Collin P, MäKI M, Koskinen O, Huhtala H, Kaukinen K, Reunala T (2014) Small bowel transglutaminase 2-specific IgA deposits in dermatitis herpetiformis. Acta Derm Venereol 94:393–397
Sardy M, Karpati S, Merkl B, Paulsson M, Smyth N (2002) Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J Exp Med 195:747–757
Hadjivassiliou M, Aeschlimann P, Strigun A, Sanders DS, Woodroofe N, Aeschlimann D (2008) Autoantibodies in gluten ataxia recognize a novel neuronal transglutaminase. Ann Neurol 64:332–343
Hadjivassiliou M, Sanders DS, Grünewald RA, Woodroofe N, Boscolo S, Aeschlimann D (2010) Gluten sensitivity: from gut to brain. Lancet Neurol 9:318–330
Halfdanarson TR, Litzow MR, Murray JA (2007) Hematologic manifestations of celiac disease. Blood 109:412–421
Bardella MT, Vecchi M, Conte D, Del Ninno E, Fraquelli M, Pacchetti S, Minola E, Landoni M, Cesana BM, De Franchis R (1999) Chronic unexplained hypertransaminasemia may be caused by occult celiac disease. Hepatology 29:654–657
Kaukinen K, Halme L, Collin P, Färkkilä M, Mäki M, Vehmanen P, Partanen J, Höckerstedt K (2002) Celiac disease in patients with severe liver disease: gluten-free diet may reverse hepatic failure. Gastroenterology 122:881–888
Mazure R, Vazquez H, Gonzalez D, Mautalen C, Pedreira S, Boerr L, Bai JC (1994) Bone mineral affection in asymptomatic adult patients with celiac disease. Am J Gastroenterol 89:2130–2134
Vazquez H, Mazure R, Gonzalez D, Flores D, Pedreira S, Niveloni S, Smecuol E, Mauriño E, Bai JC (2000) Risk of fractures in celiac disease patients: a cross-sectional, case-control study. Am J Gastroenterol 95:183–189
Saccone G, Berghella V, Sarno L, Maruotti GM, Cetin I, Greco L, Khashan AS, McCarthy F, Martinelli D, Fortunato F (2015) Celiac disease and obstetric complications: a systematic review and metaanalysis. Obstet Gynecol 4:225–234
Ventura A, Magazzù G, Greco L (1999) Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. Gastroenterology 117:297–303
Viljamaa M, Kaukinen K, Huhtala H, Kyrönpalo S, Rasmussen M, Collin P (2005) Coeliac disease, autoimmune diseases and gluten exposure. Scand J Gastroenterol 40:437–443
Mårild K, Stephansson O, Grahnquist L, Cnattingius S, Söderman G, Ludvigsson JF (2013) Down syndrome is associated with elevated risk of celiac disease: a nationwide case-control study. J Pediatr 163:237–242
Frost AR, Band MM, Conway GS (2009) Serological screening for coeliac disease in adults with Turner’s syndrome: prevalence and clinical significance of endomysium antibody positivity. Eur J Endocrinol 160:675–679
Walker-Smith J, Guandalini S, Schmitz J, Shmerling D, Visakorpi J (1990) Revised criteria for diagnosis of coeliac disease. Arch Dis Child 65:909–911
Taavela J, Koskinen O, Huhtala H, Lähdeaho M, Popp A, Laurila K, Collin P, Kaukinen K, Kurppa K, Mäki M (2013) Validation of morphometric analyses of small-intestinal biopsy readouts in celiac disease. PLoS One 8:e76163
Salmi T, Collin P, Reunala T, Mäki M, Kaukinen K (2010) Diagnostic methods beyond conventional histology in coeliac disease diagnosis. Dig Liver Dis 42:28–32
Freeman HJ (2004) REVIEW: adult celiac disease and the severe “flat” small bowel biopsy lesion. Dig Dis Sci 49:535–545
Marsh MN (1992) Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (“celiac sprue”). Gastroenterology 102:330–354
Kurppa K, Collin P, Viljamaa M, Haimila K, Saavalainen P, Partanen J, Laurila K, Huhtala H, Paasikivi K, Mäki M (2009) Diagnosing mild enteropathy celiac disease: a randomized, controlled clinical study. Gastroenterology 136:816–823
Kaukinen K, Lindfors K, Collin P, Koskinen O, Maki M (2010) Coeliac disease—a diagnostic and therapeutic challenge. Clin Chem Lab Med 48:1205–1216
Walker MM, Murray JA, Ronkainen J, Aro P, Storskrubb T, D'Amato M, Lahr B, Talley NJ, Agreus L (2010) Detection of celiac disease and lymphocytic enteropathy by parallel serology and histopathology in a population-based study. Gastroenterology 139:112–119
Järvinen TT, Kaukinen K, Laurila K, Kyrönpalo S, Rasmussen M, Mäki M, Korhonen H, Reunala T, Collin P (2003) Intraepithelial lymphocytes in celiac disease. Am J Gastroenterol 98:1332–1337
Leffler DA, Schuppan D (2010) Update on serologic testing in celiac disease. Am J Gastroenterol 105:2520–2524
Kaukinen K, Collin P, Laurila K, Kaartinen T, Partanen J, Mäki M (2007) Resurrection of gliadin antibodies in coeliac disease. Deamidated gliadin peptide antibody test provides additional diagnostic benefit. Scand J Gastroenterol 42:1428–1433
Kurppa K, Lindfors K, Collin P, Saavalainen P, Partanen J, Haimila K, Huhtala H, Laurila K, Maki M, Kaukinen K (2011) Antibodies against deamidated gliadin peptides in early-stage celiac disease. J Clin Gastroenterol 45:673–678
Dahle C, Hagman A, Ignatova S, Ström M (2010) Antibodies against deamidated gliadin peptides identify adult coeliac disease patients negative for antibodies against endomysium and tissue transglutaminase. Aliment Pharmacol Ther 32:254–260
Seah P, Fry L, Rossiter M, Hopfbrand A, Holborow E (1971) Anti-reticulin antibodies in childhood coeliac disease. Lancet 298:681–682
Chorzelski T, Beutner E, Sulej J, Tchorzewska H, Jablonska S, Kumar V, Kapuscinska A (1984) IgA anti-endomysium antibody. A new immunological marker of dermatitis herpetiformis and coeliac disease. Br J Dermatol 111:395–402
Ladinser B, Rossipal E, Pittschieler K (1994) Endomysium antibodies in coeliac disease: an improved method. Gut 35:776–778
Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken E, Schuppan D (1997) Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med 3:797–801
Maki M, Holm K, Hallstrom O, Collin P, Viander M, Savilahti E, Lipsanen V, Koskimies S (1991) Serological markers and HLA genes among healthy first-degree relatives of patients with coeliac disease. Lancet 338:1350–1353
Taavela J, Kurppa K, Collin P, Lähdeaho M, Salmi T, Saavalainen P, Haimila K, Huhtala H, Laurila K, Sievänen H (2013) Degree of damage to the small bowel and serum antibody titers correlate with clinical presentation of patients with celiac disease. Clin Gastroenterol Hepatol 11:166–171
Husby S, Koletzko S, Korponay-Szabo IR, Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski D, Catassi C, Lelgeman M, Maki M, Ribes-Koninckx C, Ventura A, Zimmer KP, ESPGHAN Working Group on Coeliac Disease Diagnosis, ESPGHAN Gastroenterology Committee & European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (2012) European Society for Pediatric Gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 54:136–160
Nemec G, Ventura A, Stefano M, Di Leo G, Baldas V, Tommasini A, Ferrara F, Taddio A, Citta A, Sblattero D (2006) Looking for celiac disease: diagnostic accuracy of two rapid commercial assays. Am J Gastroenterol 101:1597–1600
Popp A, Jinga M, Jurcut C, Balaban V, Bardas C, Laurila K, Vasilescu F, Ene A, Anca I, Maki M (2013) Fingertip rapid point-of-care test in adult case-finding in coeliac disease. BMC Gastroenterol 13:115
Mooney PD, Wong SH, Johnston AJ, Kurien M, Avgerinos A, Sanders DS (2015) Increased detection of celiac disease with measurement of deamidated gliadin peptide antibody before endoscopy. Clin Gastroenterol Hepatol 13:1278–1284
Marzari R, Sblattero D, Florian F, Tongiorgi E, Not T, Tommasini A, Ventura A, Bradbury A (2001) Molecular dissection of the tissue transglutaminase antoantibody response in celiac disease. J Immunol 166:4170–4176
Sblattero D, Ventura A, Tommasini A, Cattin L, Martelossi S, Florian F, Marzari R, Bradbury A, Not T (2006) Cryptic gluten intolerance in type 1 diabetes: identifying suitable candidates for a gluten free diet. Gut 55:133–134
Shiner M, Ballard J (1972) Antigen-antibody reactions in jejunal mucosa in childhood coeliac disease after gluten challenge. Lancet 299:1202–1205
Kárpáti S, Kósnai I, Török É, Kovács JB (1988) Immunoglobulin a deposition in jejunal mucosa of children with dermatitis herpetiformis. J Investig Dermatol 91:336–339
Korponay-Szabo I, Halttunen T, Szalai Z, Laurila K, Kiraly R, Kovacs J, Fesus L, Maki M (2004) In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut 53:641–648
Kaukinen K, Peräaho M, Collin P, Partanen J, Woolley N, Kaartinen T, Nuutinen T, Halttunen T, Mäki M, Korponay-Szabo I (2005) Small-bowel mucosal transglutaminase 2-specific IgA deposits in coeliac disease without villous atrophy: a prospective and randomized clinical study. Scand J Gastroenterol 40:564–572
Salmi TT, Collin P, Korponay-Szabo IR, Laurila K, Partanen J, Huhtala H, Kiraly R, Lorand L, Reunala T, Maki M, Kaukinen K (2006) Endomysial antibody-negative coeliac disease: clinical characteristics and intestinal autoantibody deposits. Gut 55:1746–1753
Jv M (1969) Granular deposits of immunoglobulins in the skin of patients with dermatitis herpetiformis. An immunofluorescent study. Br J Dermatol 81:493–503
Heil PM, Volc-Platzer B, Karlhofer F, Gebhart W, Huber W, Benesch T, Vogelsang H, Stingl G (2005) Transglutaminases as diagnostically relevant autoantigens in patients with gluten sensitivity. J Dtsch Dermatol Ges 3:974–978
Marietta EV, Camilleri MJ, Castro LA, Krause PK, Pittelkow MR, Murray JA (2008) Transglutaminase autoantibodies in dermatitis herpetiformis and celiac sprue. J Investig Dermatol 128:332–335
Hull CM, Liddle M, Hansen N, Meyer L, Schmidt L, Taylor T, Jaskowski T, Hill H, Zone J (2008) Elevation of IgA anti-epidermal transglutaminase antibodies in dermatitis herpetiformis. Br J Dermatol 159:120–124
Borroni G, Biagi F, Ciocca O, Vassallo C, Carugno A, Cananzi R, Campanella J, Bianchi P, Brazzelli V, Corazza G (2013) IgA anti-epidermal transglutaminase autoantibodies: a sensible and sensitive marker for diagnosis of dermatitis herpetiformis in adult patients. J Eur Acad Dermatol Venereol 27:836–841
Jaskowski TD, Hamblin T, Wilson AR, Hill HR, Book LS, Meyer LJ, Zone JJ, Hull CM (2009) IgA anti-epidermal transglutaminase antibodies in dermatitis herpetiformis and pediatric celiac disease. J Investig Dermatol 129:2728–2730
Janatuinen EK, Kemppainen TA, Julkunen RJ, Kosma VM, Maki M, Heikkinen M, Uusitupa MI (2002) No harm from five year ingestion of oats in coeliac disease. Gut 50:332–335
Kaukinen K, Collin P, Huhtala H, Mäki M (2013) Long-term consumption of oats in adult celiac disease patients. Nutrients 5:4380–4389
Hopman EG, von Blomberg ME, Batstra MR, Morreau H, Dekker FW, Koning F, Lamers CB, Mearin ML (2008) Gluten tolerance in adult patients with celiac disease 20 years after diagnosis? Eur J Gastroenterol Hepatol 20:423–429
Bardella M, Fredella C, Trovato C, Ermacora E, Cavalli R, Saladino V, Prampolini L (2003) Long-term remission in patients with dermatitis herpetiformis on a normal diet. Br J Dermatol 149:968–971
Paek SY, Steinberg SM, Katz SI (2011) Remission in dermatitis herpetiformis: a cohort study. Arch Dermatol 147:301–305
Tio M, Cox M, Eslick G (2012) Meta-analysis: coeliac disease and the risk of all-cause mortality, any malignancy and lymphoid malignancy. Aliment Pharmacol Ther 35:540–551
Hervonen K, Vornanen M, Kautiainen H, Collin P, Reunala T (2005) Lymphoma in patients with dermatitis herpetiformis and their first-degree relatives. Br J Dermatol 152:82–86
Blazina Š, Bratanič N, Čampa AŠ (2010) Bone mineral density and importance of strict gluten-free diet in children and adolescents with celiac disease. Bone 47:598–603
See JA, Kaukinen K, Makharia GK, Gibson PR, Murray JA (2015) Practical insights into gluten-free diets. Nat Rev Gastroenterol Hepatol 12:580–591
van Gils T, Nijeboer P, van Wanrooij RL, Bouma G, Mulder CJ (2015) Mechanisms and management of refractory coeliac disease. Nat Rev Gastroenterol Hepatol 12:572–579
Ilus T, Kaukinen K, Virta L, Huhtala H, Mäki M, Kurppa K, Heikkinen M, Heikura M, Hirsi E, Jantunen K (2014) Refractory coeliac disease in a country with a high prevalence of clinically-diagnosed coeliac disease. Aliment Pharmacol Ther 39:418–425
Sanchez M, Mohaidle A, Baistrocchi A, Matoso D, Vázquez H, González A, Mazure R, Maffei E, Ferrari G, Smecuol E (2011) Risk of fracture in celiac disease: gender, dietary compliance, or both. World J Gastroenterol 17:3035–3042
Norström F, Sandström O, Lindholm L, Ivarsson A (2012) A gluten-free diet effectively reduces symptoms and health care consumption in a Swedish celiac disease population. BMC Gastroenterol 12:1
Lerner A (2010) New therapeutic strategies for celiac disease. Autoimmun Rev 9:144–147
Sulic A, Kurppa K, Rauhavirta T, Kaukinen K, Lindfors K (2015) Transglutaminase as a therapeutic target for celiac disease. Expert Opin Ther Targets 19:335–348
Shan L, Molberg O, Parrot I, Hausch F, Filiz F, Gray G, Sollid L, Khosla C (2002) Structural basis for gluten intolerance in celiac sprue. Science 297:2275–2279
Quarsten H, Molberg Ø, Fugger L, McAdam SN, Sollid LM (1999) HLA binding and T cell recognition of a tissue transglutaminase-modified gliadin epitope. Eur J Immunol 29:2506–2514
Vader L, de Ru A, van der Wal Y, Kooy Y, Benckhuijsen W, Mearin M, Drijfhout J, van Veelen P, Koning F (2002) Specificity of tissue transglutaminase explains cereal toxicity in celiac disease. J Exp Med 195:643–649
Molberg O, Mcadam S, Korner R, Quarsten H, Kristiansen C, Madsen L, Fugger L, Scott H, Noren O, Roepstorff P, Lundin K, Sjostrom H, Sollid L (1998) Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease RID G-8565-2011. Nat Med 4:713–717
Dahal-Koirala S, Risnes L, Christophersen A, Sarna V, Lundin KE, Sollid L, Qiao S (2016) TCR sequencing of single cells reactive to DQ2. 5-glia-α2 and DQ2. 5-glia-ω2 reveals clonal expansion and epitope-specific V-gene usage. Mucosal Immunol. doi:10.1038/mi.2015.147
Qiao SW, Christophersen A, Lundin KE, Sollid LM (2014) Biased usage and preferred pairing of alpha- and beta-chains of TCRs specific for an immunodominant gluten epitope in coeliac disease. Int Immunol 26:13–19
Qiao SW, Raki M, Gunnarsen KS, Loset GA, Lundin KE, Sandlie I, Sollid LM (2011) Posttranslational modification of gluten shapes TCR usage in celiac disease. J Immunol 187:3064–3071
Petersen J, Montserrat V, Mujico JR, Loh KL, Beringer DX, van Lummel M, Thompson A, Mearin ML, Schweizer J, Kooy-Winkelaar Y (2014) T-cell receptor recognition of HLA-DQ2–gliadin complexes associated with celiac disease. Nat Struct Mol Biol 21:480–488
Fleckenstein B, Qiao SW, Larsen MR, Jung G, Roepstorff P, Sollid LM (2004) Molecular characterization of covalent complexes between tissue transglutaminase and gliadin peptides. J Biol Chem 279:17607–17616
du Pré MF, Sollid LM (2015) T-cell and B-cell immunity in celiac disease. Best Pract Res Clin Gastroenterol 29:413–423
Di Niro R, Mesin L, Zheng N, Stamnaes J, Morrissey M, Lee J, Huang M, Iversen R, du Pre MF, Qiao S, Lundin KEA, Wilson PC, Sollid LM (2012) High abundance of plasma cells secreting transglutaminase 2-specific IgA autoantibodies with limited somatic hypermutation in celiac disease intestinal lesions. Nat Med 18:441–445
Villanacci V, Not T, Sblattero D, Gaiotto T, Chirdo F, Galletti A, Bassotti G (2009) Mucosal tissue transglutaminase expression in celiac disease. J Cell Mol Med 13:334–340
Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD (1988) Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 29:1035–1041
Rauhavirta T, Qiao S, Jiang Z, Myrsky E, Loponen J, Korponay-Szabo IR, Salovaara H, Garcia-Horsman JA, Venalainen J, Mannisto PT, Collighan R, Mongeot A, Griffin M, Maki M, Kaukinen K, Lindfors K (2011) Epithelial transport and deamidation of gliadin peptides: a role for coeliac disease patient immunoglobulin a. Clin Exp Immunol 164:127–136
Thomazy VA, Vega F, Medeiros LJ, Davies PJ, Jones D (2003) Phenotypic modulation of the stromal reticular network in normal and neoplastic lymph nodes: tissue transglutaminase reveals coordinate regulation of multiple cell types. Am J Pathol 163:165–174
Mention J, Ahmed MB, Bègue B, Barbe U, Verkarre V, Asnafi V, Colombel J, Cugnenc P, Ruemmele FM, Mcintyre E (2003) Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology 125:730–745
Barone MV, Zanzi D, Maglio M, Nanayakkara M, Santagata S, Lania G, Miele E, Ribecco MTS, Maurano F, Auricchio R (2011) Gliadin-mediated proliferation and innate immune activation in celiac disease are due to alterations in vesicular trafficking. PLoS One 6:e17039
Hue S, Mention J, Monteiro R, Zhang S, Cellier C, Schmitz J, Verkarre V, Fodil N, Bahram S, Cerf-Bensussan N, Caillat-Zucman S (2004) A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 21:367–377
Roberts AI, Lee L, Schwarz E, Groh V, Spies T, Ebert EC, Jabri B (2001) NKG2D receptors induced by IL-15 costimulate CD28-negative effector CTL in the tissue microenvironment. J Immunol 167:5527–5530
Setty M, Discepolo V, Abadie V, Kamhawi S, Mayassi T, Kent A, Ciszewski C, Maglio M, Kistner E, Bhagat G (2015) Distinct and synergistic contributions of epithelial stress and adaptive immunity to functions of intraepithelial killer cells and active celiac disease. Gastroenterology 149:681–691
Meresse B, Curran SA, Ciszewski C, Orbelyan G, Setty M, Bhagat G, Lee L, Tretiakova M, Semrad C, Kistner E, Winchester RJ, Braud V, Lanier LL, Geraghty DE, Green PH, Guandalini S, Jabri B (2006) Reprogramming of CTLs into natural killer-like cells in celiac disease. J Exp Med 203:1343–1355
Steinsbø Ø, Dunand CJH, Huang M, Mesin L, Salgado-Ferrer M, Lundin KE, Jahnsen J, Wilson PC, Sollid LM (2014) Restricted VH/VL usage and limited mutations in gluten-specific IgA of coeliac disease lesion plasma cells. Nat Commun 5:4041
Sblattero D, Florian F, Azzoni E, Zyla T, Park M, Baldas V, Not T, Ventura A, Bradbury A, Marzari R (2002) The analysis of the fine specificity of celiac disease antibodies using tissue transglutaminase fragments. Eur J Biochem 269:5175–5181
Comerford R, Byrne G, Feighery C, Kelly J (2012) Binding of autoantibodies to the core region of tissue transglutaminase is a feature of paediatric coeliac disease. J Pediatr Gastroenterol Nutr 55:445–450
Simon-Vecsei Z, Kiraly R, Bagossi P, Toth B, Dahlbom I, Caja S, Csosz E, Lindfors K, Sblattero D, Nemes E, Maki M, Fesus L, Korponay-Szabo IR (2012) A single conformational transglutaminase 2 epitope contributed by three domains is critical for celiac antibody binding and effects. Proc Natl Acad Sci U S A 109:431–436
Iversen R, Di Niro R, Stamnaes J, Lundin KE, Wilson PC, Sollid LM (2013) Transglutaminase 2-specific autoantibodies in celiac disease target clustered, N-terminal epitopes not displayed on the surface of cells. J Immunol 190:5981–5991
Farrace MG, Picarelli A, Di Tola M, Sabbatella L, Marchione OP, Ippolito G, Piacentini M (2001) Presence of anti-“tissue” transglutaminase antibodies in inflammatory intestinal diseases: an apoptosis-associated event? Cell Death Differ 8:767–770
Lidar M, Langevitz P, Barzilai O, Ram M, Porat-Katz B, Bizzaro N, Tonutti E, Maieron R, Chowers Y, Bar-Meir S (2009) Infectious serologies and autoantibodies in inflammatory bowel disease. Ann N Y Acad Sci 1173:640–648
Pereda I, Bartolomé-Pacheco MJ, Martín M, López-Escribano H, Echevarría S, López-Hoyos M (2001) Antitissue transglutaminase antibodies in HIV infection and effect of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 27:507–508
Peracchi M, Trovato C, Longhi M, Gasparin M, Conte D, Tarantino C, Prati D, Bardella MT (2002) Tissue transglutaminase antibodies in patients with end-stage heart failure. Am J Gastroenterol 97:2850–2854
Sárdy M, Csikós M, Geisen C, Preisz K, Kornseé Z, Tomsits E, Töx U, Hunzelmann N, Wieslander J, Kárpáti S (2007) Tissue transglutaminase ELISA positivity in autoimmune disease independent of gluten-sensitive disease. Clin Chim Acta 376:126–135
Kiraly R, Vecsei Z, Demenyi T, Korponay-Szabo I, Fesus L (2006) Coeliac autoantibodies can enhance transamidating and inhibit GTPase activity of tissue transglutaminase: dependence on reaction environment and enzyme fitness. J Autoimmun 26:278–287
Myrsky E, Caja S, Simon-Vecsei Z, Korponay-Szabo IR, Nadalutti C, Collighan R, Mongeot A, Griffin M, Maki M, Kaukinen K, Lindfors K (2009) Celiac disease IgA modulates vascular permeability in vitro through the activity of transglutaminase 2 and RhoA. Cell Mol Life Sci 66:3375–3385
Dieterich W, Trapp D, Esslinger B, Leidenberger M, Piper J, Hahn E, Schuppan D (2003) Autoantibodies of patients with coeliac disease are insufficient to block tissue transglutaminase activity. Gut 52:1562–1566
Byrne G, Feighery C, Jackson J, Kelly J (2010) Coeliac disease autoantibodies mediate significant inhibition of tissue transglutaminase. Clin Immunol 136:426–431
Esposito C, Paparo F, Caputo I, Rossi M, Maglio M, Sblattero D, Not T, Porta R, Auricchio S, Marzari R, Troncone R (2002) Anti-tissue transglutaminase antibodies from coeliac patients inhibit transglutaminase activity both in vitro and in situ. Gut 51:177–181
Halttunen T, Maki M (1999) Serum immunoglobulin a from patients with celiac disease inhibits human T84 intestinal crypt epithelial cell differentiation. Gastroenterology 116:566–572
Barone MV, Caputo I, Ribecco MT, Maglio M, Marzari R, Sblattero D, Troncone R, Auricchio S, Esposito C (2007) Humoral immune response to tissue transglutaminase is related to epithelial cell proliferation in celiac disease. Gastroenterology 132:1245–1253
Teesalu K, Panarina M, Uibo O, Uibo R, Utt M (2012) Autoantibodies from patients with celiac disease inhibit transglutaminase 2 binding to heparin/heparan sulfate and interfere with intestinal epithelial cell adhesion. Amino Acids 42:1055–1064
Zanoni G, Navone R, Lunardi C, Tridente G, Bason C, Sivori S, Beri R, Dolcino M, Valletta E, Corrocher R, Puccetti A (2006) In celiac disease, a subset of autoantibodies against transglutaminase binds toll-like receptor 4 and induces activation of monocytes. PLoS Med 3:1637–1653
Lebreton C, Ménard S, Abed J, Moura IC, Coppo R, Dugave C, Monteiro RC, Fricot A, Traore MG, Griffin M, Cellier C, Malamut G, Cerf-Bensussan N, Heyman M (2012) Interactions among secretory immunoglobulin a, CD71, and transglutaminase-2 affect permeability of intestinal epithelial cells to gliadin peptides. Gastroenterology 143(3):698–707
Ménard S, Lebreton C, Schumann M, Matysiak-Budnik T, Dugave C, Bouhnik Y, Malamut G, Cellier C, Allez M, Crenn P (2012) Paracellular versus transcellular intestinal permeability to gliadin peptides in active celiac disease. Am J Pathol 180:608–615
Matysiak-Budnik T, Moura IC, Arcos-Fajardo M, Lebreton C, Menard S, Candalh C, Ben-Khalifa K, Dugave C, Tamouza H, van Niel G, Bouhnik Y, Lamarque D, Chaussade S, Malamut G, Cellier C, Cerf-Bensussan N, Monteiro RC, Heyman M (2008) Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J Exp Med 205:143–154
Nadalutti CA, Korponay-Szabo IR, Kaukinen K, Griffin M, Mäki M, Lindfors K (2014) Celiac disease patient IgA antibodies induce endothelial adhesion and cell polarization defects via extracellular transglutaminase 2. Cell Mol Life Sci 71:1315–1326
Kalliokoski S, Sulic A, Korponay-Szabó IR, Szondy Z, Frias R, Perez MA, Martucciello S, Roivainen A, Pelliniemi LJ, Esposito C (2013) Celiac disease-specific TG2-targeted autoantibodies inhibit angiogenesis ex vivo and in vivo in mice by interfering with endothelial cell dynamics. PLoS One 8:e65887
Myrsky E, Kaukinen K, Syrjanen M, Korponay-Szabo IR, Maki M, Lindfors K (2008) Coeliac disease-specific autoantibodies targeted against transglutaminase 2 disturb angiogenesis. Clin Exp Immunol 152:111–119
Cooke WT, Holmes GKT (1984) Coeliac disease. Churchill Livingstone, London
Freitag T, Schulze-Koops H, Niedobitek G, Melino G, Schuppan D (2004) The role of the immune response against tissue transglutaminase in the pathogenesis of coeliac disease. Autoimmun Rev 3:13–20
Di Niro R, Sblattero D, Florian F, Stebel M, Zentilin L, Giacca M, Villanacci V, Galletti A, Not T, Ventura A (2008) Anti-idiotypic response in mice expressing human autoantibodies. Mol Immunol 45:1782–1791
Kalliokoski S, Caja S, Frias R, Laurila K, Koskinen O, Niemelä O, Mäki M, Kaukinen K, Korponay-Szabó IR, Lindfors K (2015) Injection of celiac disease patient sera or immunoglobulins to mice reproduces a condition mimicking early developing celiac disease. J Mol Med 93:51–62
Hadjivassiliou M, Maki M, Sanders D, Williamson C, Grunewald R, Woodroofe N, Korponay-Szabo I (2006) Autoantibody targeting of brain and intestinal transglutaminase in gluten ataxia. Neurology 66:373–377
Boscolo S, Lorenzon A, Sblattero D, Florian F, Stebel M, Marzari R, Not T, Aeschlimann D, Ventura A, Hadjivassiliou M (2010) Anti transglutaminase antibodies cause ataxia in mice. PLoS One 5:e9698
Smecuol E, Mauriño E, Vazquez H, Pedreira S, Niveloni S, Mazure R, Boerr L, Bai JC (1996) Gynaecological and obstetric disorders in coeliac disease: frequent clinical onset during pregnancy or the puerperium. Eur J Gastroenterol Hepatol 8:63–68
Lasa JS, Zubiaurre I, Soifer LO (2014) Risk of infertility in patients with celiac disease: a meta-analysis of observational studies. Arq Gastroenterol 51:144–150
Anjum N, Baker PN, Robinson NJ, Aplin JD (2009) Maternal celiac disease autoantibodies bind directly to syncytiotrophoblast and inhibit placental tissue transglutaminase activity. Reprod Biol Endocrinol 7:16
Sóñora C, Calo G, Fraccaroli L, Pérez-Leirós C, Hernández A, Ramhorst R (2014) Tissue transglutaminase on trophoblast cells as a possible target of autoantibodies contributing to pregnancy complications in celiac patients. Am J Reprod Immunol 72:485–495
Acknowledgments
This work was supported by funding from the Academy of Finland, the Competitive State Research Financing of the Expert Responsibility Areas of Tampere University Hospital (Grant 9T058), the Finnish Medical Foundation, the Sigrid Juselius Foundation, and the Päivikki and Sakari Sohlberg Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rauhavirta, T., Hietikko, M., Salmi, T. et al. Transglutaminase 2 and Transglutaminase 2 Autoantibodies in Celiac Disease: a Review. Clinic Rev Allerg Immunol 57, 23–38 (2019). https://doi.org/10.1007/s12016-016-8557-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-016-8557-4