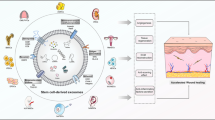
Abstract
Background
Although mesenchymal stem cells (MSCs) are used as therapeutic agents for skin injury therapy, few studies have reported the effects of dosing duration and delivery frequency on wound healing. In addition, before the clinical application of MSCs, it is important to assess whether their usage might influence tumor occurrence.
Methods
We described the metabolic patterns of subcutaneous injection of hUC-MSCs using fluorescence tracing and qPCR methods and applied them to the development of drug delivery strategies for promoting wound healing.
Results
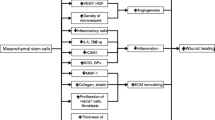
(i) We developed cGMP-compliant hUC-MSC products with critical quality control points for wound healing; (ii) The products did not possess any tumorigenic or tumor-promoting/inhibiting ability in vivo; (iii) Fluorescence tracing and qPCR analyses showed that the subcutaneous application of hUC-MSCs did not result in safety-relevant biodistribution or ectopic migration; (iv) Reinjecting hUC-MSCs after significant consumption significantly improved reepithelialization and dermal regeneration.
Conclusions
Our findings provided a reference for controlling the quality of MSC products used for wound healing and highlighted the importance of delivery time and frequency for designing in vivo therapeutic studies.
Graphical Abstract







Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
References
Bowers, S., & Franco, E. (2020). Chronic wounds: Evaluation and management. American Family Physician, 101(3), 159–166.
Bjarnsholt, T., et al. (2008). Why chronic wounds will not heal: A novel hypothesis. Wound Repair and Regeneration, 16(1), 2–10.
Bian, D., et al. (2022). The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: A comprehensive review. Stem Cell Research & Therapy, 13(1), 24.
Tottoli, E. M., et al. (2020). Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics, 12(8), 735.
Bray, E. R., Kirsner, R. S., & Badiavas, E. V. (2022). Mesenchymal stem cell–derived extracellular vesicles as an advanced therapy for chronic wounds. Cold Spring Harbor Perspectives in Biology, 14(10), a041227.
Schneider, C., Stratman, S., & Kirsner, R. S. (2021). Lower extremity ulcers. Medical Clinics of North America, 105(4), 663–679.
Stupin, V., et al. (2020). The effect of inflammation on the healing process of acute skin wounds under the treatment of wounds with injections in rats. Journal of Experimental Pharmacology, 12, 409–422.
Galderisi, U., Peluso, G., & Di Bernardo, G. (2021). Clinical trials based on mesenchymal stromal cells are exponentially increasing: Where are we in recent years? Stem Cell Reviews and Reports, 18(1), 23–36.
Squillaro, T., Peluso, G., & Galderisi, U. (2016). Clinical trials with mesenchymal stem cells: An update. Cell Transplantation, 25(5), 829–848.
Vaes, B., et al. (2012). Application of MultiStem® allogeneic cells for immunomodulatory therapy: Clinical progress and pre-clinical challenges in prophylaxis for graft versus host disease. Frontiers in Immunology, 3, 345.
Konishi, A., et al. (2016). First approval of regenerative medical products under the PMD act in Japan. Cell Stem Cell, 18(4), 434–435.
Hashmi, S., et al. (2016). Survival after mesenchymal stromal cell therapy in steroid-refractory acute graft-versus-host disease: Systematic review and meta-analysis. The Lancet Haematology, 3(1), e45–e52.
Wang, X., et al. (2022). Secretome of human umbilical cord mesenchymal stem cell maintains skin homeostasis by regulating multiple skin physiological function. Cell and Tissue Research, 391(1), 111–125.
Ra, J. C., et al. (2011). Stem cell treatment for patients with autoimmune disease by systemic infusion of culture-expanded autologous adipose tissue derived mesenchymal stem cells. Journal of Translational Medicine, 9(1), 181.
Le Blanc, K., & Ringdén, O. (2007). Immunomodulation by mesenchymal stem cells and clinical experience. Journal of Internal Medicine, 262(5), 509–525.
Dai, Y., et al. (2007). Skin epithelial cells in mice from umbilical cord blood mesenchymal stem cells. Burns, 33(4), 418–428.
Lataillade, J. J., et al. (2007). New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regenerative Medicine, 2(5), 785–794.
Vojtassák, J., et al. (2006). Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neuro Endocrinology Letters, 27(Suppl 2), 134–137.
Zhang, Q.-Z., et al. (2010). Human Gingiva-derived mesenchymal stem cells elicit polarization of M2 macrophages and enhance cutaneous wound healing. Stem Cells, 28(10), 1856–1868.
Bozza, P., et al. (2008). Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE, 3(4), e1886.
Singer, N. G., & Caplan, A. I. (2011). Mesenchymal stem cells: mechanisms of inflammation. Annual Review of Pathology: Mechanisms of Disease, 6(1), 457–478.
Sasaki, M., et al. (2008). Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. The Journal of Immunology, 180(4), 2581–2587.
Altman, A. M., et al. (2008). Dermal matrix as a carrier for in vivo delivery of human adipose-derived stem cells. Biomaterials, 29(10), 1431–1442.
Shumakov, V. I., et al. (2003). Mesenchymal bone marrow stem cells more effectively stimulate regeneration of deep burn wounds than embryonic fibroblasts. Bulletin of Experimental Biology and Medicine, 136(2), 192–195.
Kwon, D. S., et al. (2008). Treatment with bone marrow-derived stromal cells accelerates wound healing in diabetic rats. International Wound Journal, 5(3), 453–463.
Wu, Y., et al. (2014). Bone marrow-derived mesenchymal stem cell attenuates skin fibrosis development in mice. International Wound Journal, 11(6), 701–710.
Han, Y., et al. (2019). Mesenchymal stem cells for regenerative medicine. Cells, 8(8), 886.
Körbling, M., et al. (2002). Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. New England Journal of Medicine, 346(10), 738–746.
Murata, H., et al. (2006). Donor-derived cells and human graft-versus-host disease of the skin. Blood, 109(6), 2663–2665.
Kumamoto, T., et al. (2003). Hair follicles serve as local reservoirs of skin mast cell precursors. Blood, 102(5), 1654–1660.
Lako, M., et al. (2002). Hair follicle dermal cells repopulate the mouse haematopoietic system. Journal of Cell Science, 115(20), 3967–3974.
Cho, D.-I., et al. (2014). Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Experimental & Molecular Medicine, 46(1), e70–e70.
Dameshghi, S., et al. (2016). Mesenchymal stem cells alter macrophage immune responses to Leishmania major infection in both susceptible and resistance mice. Immunology Letters, 170, 15–26.
Hu, Y., et al. (2016). Mesenchymal stem cell-educated macrophages ameliorate LPS-induced systemic response. Mediators of Inflammation, 2016, 1–12.
Jackson, W. M., Nesti, L. J., & Tuan, R. S. (2012). Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem Cell Research & Therapy, 3(3), 20.
Kanji, S., & Das, H. (2017). Advances of stem cell therapeutics in cutaneous wound healing and regeneration. Mediators of Inflammation, 2017, 1–14.
Seo, B. F., & Jung, S.-N. (2016). The immunomodulatory effects of mesenchymal stem cells in prevention or treatment of excessive scars. Stem Cells International, 2016, 1–8.
Wang, X. I. N., et al. (2016). Enhanced expression of polysialic acid correlates with malignant phenotype in breast cancer cell lines and clinical tissue samples. International Journal of Molecular Medicine, 37(1), 197–206.
André, F., et al. (2015). Promotion of cell migration by Neural Cell Adhesion Molecule (NCAM) is enhanced by PSA in a polysialyltransferase-specific manner. PLoS ONE, 10(4), e0124237.
Zeng, C., et al. (2010). Evaluation of 5-ethynyl-2′-deoxyuridine staining as a sensitive and reliable method for studying cell proliferation in the adult nervous system. Brain Research, 1319, 21–32.
Livak, K. J., & Schmittgen, T. D. L. J. M. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods, 25(4), 402–408.
Birch, M., Tomlinson, A., & Ferguson, M. W. (2005). Animal models for adult dermal wound healing. Methods in Molecular Medicine, 117, 223–235.
Duffield, J. S., et al. (2013). Host responses in tissue repair and fibrosis. Annual Review of Pathology: Mechanisms of Disease, 8(1), 241–276.
Galipeau, J., et al. (2016). International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy, 18(2), 151–159.
Krampera, M., et al. (2013). Immunological characterization of multipotent mesenchymal stromal cells–the International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy, 15(9), 1054–1061.
Naji, A., et al. (2019). Biological functions of mesenchymal stem cells and clinical implications. Cellular and Molecular Life Sciences, 76(17), 3323–3348.
Gallant-Behm, C. L., et al. (2011). Epithelial regulation of mesenchymal tissue behavior. Journal of Investigative Dermatology, 131(4), 892–899.
Doi, H., et al. (2016). Potency of umbilical cord blood- and Wharton’s jelly-derived mesenchymal stem cells for scarless wound healing. Scientific Reports, 6(1), 18844.
Maxson, S., et al. (2012). Concise review: Role of mesenchymal stem cells in wound repair. Stem Cells Translational Medicine, 1(2), 142–149.
Sobacchi, C., et al. (2017). Soluble factors on stage to direct mesenchymal stem cells fate. Frontiers in Bioengineering and Biotechnology, 5, 32.
Joel, M. D. M., et al. (2019). MSC: Immunoregulatory effects, roles on neutrophils and evolving clinical potentials. American Journal of Translational Research, 11(6), 3890–3904.
Gurtner, G. C., et al. (2008). Wound repair and regeneration. Nature, 453(7193), 314–321.
Rhett, J. M., et al. (2008). Novel therapies for scar reduction and regenerative healing of skin wounds. Trends in Biotechnology, 26(4), 173–180.
Werner, S., & Grose, R. (2003). Regulation of wound healing by growth factors and cytokines. Physiological Reviews, 83(3), 835–870.
Guo, S.-C., et al. (2017). Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics, 7(1), 81–96.
Lyamina, S., et al. (2023). Mesenchymal stromal cells as a driver of inflammaging. International Journal of Molecular Sciences, 24(7), 6372.
Zangi, L., et al. (2009). Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells, 27(11), 2865–2874.
Vilalta, M., et al. (2008). Biodistribution, long-term survival, and safety of human adipose tissue-derived mesenchymal stem cells transplanted in nude mice by high sensitivity non-invasive bioluminescence imaging. Stem Cells and Development, 17(5), 993–1004.
Gholamrezanezhad, A., et al. (2011). In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nuclear Medicine and Biology, 38(7), 961–967.
Djouad, F., et al. (2005). Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor α in collagen-induced arthritis. Arthritis & Rheumatism, 52(5), 1595–1603.
Zhuang, W. Z., et al. (2021). Mesenchymal stem/stromal cell-based therapy: Mechanism, systemic safety and biodistribution for precision clinical applications. Journal of Biomedical Science, 28(1), 28.
Wu, C., et al. (2014). Polymeric vector-mediated gene transfection of MSCs for dual bioluminescent and MRI tracking in vivo. Biomaterials, 35(28), 8249–8260.
Schächinger, V., et al. (2008). Pilot trial on determinants of progenitor cell recruitment to the infarcted human myocardium. Circulation, 118(14), 1425–1432.
Gong, M., et al. (2017). Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget, 8(28), 45200–45212.
Ludwig, A.-K., et al. (2018). Precipitation with polyethylene glycol followed by washing and pelleting by ultracentrifugation enriches extracellular vesicles from tissue culture supernatants in small and large scales. Journal of Extracellular Vesicles, 7(1), 1528109.
Yi, Y. W., et al. (2020). Advances in analysis of biodistribution of exosomes by molecular imaging. International Journal of Molecular Sciences, 21(2), 665.
Wang, L.-L., et al. (2016). Pharmacological activation of cannabinoid 2 receptor attenuates inflammation, fibrogenesis, and promotes re-epithelialization during skin wound healing. European Journal of Pharmacology, 786, 128–136.
Harvey, C. (2005). Wound healing. Orthopaedic Nursing, 24(2), 143–57. quiz 158-9.
Funding
This research was funded by Science and Technology SMEs Innovation Capacity Improvement Project of Shandong Province (Grant No. 2022TSGC1004); China National Key R&D Program during the 14th Five-year Plan Period (Grant No. 2021YFA1101500).
Author information
Authors and Affiliations
Contributions
Conceptualization, W.X. and T.Y.; methodology, W.QH., W.X., M.LJ.; validation, W.QH. and T.RF.; investigation, W.X.; data curation, W.QH. and G.HZ.; writing—W.X. and T.ZQ.; supervision, T.Y. and W.X.; project administration, W.X. and T.Y. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Human umbilical cords were harvested with written informed consent from the donor. All procedures performed involving human participants in experiments were carried out per ethical standards of the institutional and/or national research committee and the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The animal study was carried out in accordance with the commendations of the Institutional Animal Care and Use Committee of Northwest University. The protocol was approved by the Institutional Animal Care and Use Committee of Northwest University.
Consent to Participate
The patients/participants provided their written informed consent to participate in this study.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.


Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Wang, Q., Meng, L. et al. Biodistribution-based Administration of cGMP-compliant Human Umbilical Cord Mesenchymal Stem Cells Affects the Therapeutic Effect of Wound Healing. Stem Cell Rev and Rep 20, 329–346 (2024). https://doi.org/10.1007/s12015-023-10644-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10644-9