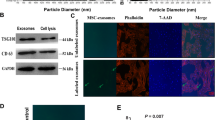
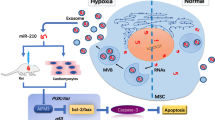
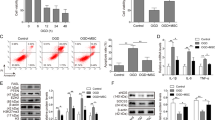
Abstract
Stem cell therapy provides a hope to no option heart disease patient group. Stem cells work via different mechanisms of which paracrine mechanism is reported to justify most of the effects. Therefore, identifying the control arms for paracrine cocktail production is necessary to tailor stem cell functions in disease contextual manner. In this study, we describe a novel paracrine cocktail regulatory axis, in stem cells, to enhance their cardioprotective abilities. We identified that HSF1 knockout resulted in reduced cardiac regenerative abilities of mesenchymal stem cells (MSCs) while its overexpression had opposite effects. Altered exosome biognesis and their miRNA cargo enrichment were found to be underlying these altered regenerative abilities. Decreased production of exosomes by MSCs accompanied their loss of HSF1 and vice versa. Moreover, the exosomes derived from HSF1 depleted MSCs showed significantly reduced candidate miRNA expression (miR-145, miR-146, 199-3p, 199b and miR-590) compared to those obtained from HSF1 overexpressing MSCs. We further discovered that HSF1 mediates miRNAs’ enrichment into exosomes via Y binding protein 1 (YBX1) and showed, by loss and gain of function strategies, that miRNAs’ enrichment in mesenchymal stem cell derived exosomes is deregulated with altered YBX1 expression. It was finally demonstrated that absence of YBX1 in MSCs, with normal HSF1 expression, resulted in significant accumulation of candidate miRNAs into the cells. Together, our data shows that HSF1 plays a critical role in determining the regenerative potential of stem cells. HSF1 does that by affecting exosome biogenesis and miRNA cargo sorting via regulation of YBX1 gene expression.
Graphical abstract







Similar content being viewed by others
Data availability
All the data related to this research work has been included in the manuscript.
Code availability
Not applicable.
References
Liang, X., Ding, Y., Zhang, Y., Tse, H. F., & Lian, Q. (2014). Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant., 23(9), 1045–1059. https://doi.org/10.3727/096368913X667709
Gnecchi, M., He, H., Liang, O. D., Melo, L. G., Morello, F., Mu, H., Noiseux, N., Zhang, L., Pratt, R. E., Ingwall, J. S., & Dzau, V. J. (2005). Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med., 11(4), 367–368. https://doi.org/10.1038/nm0405-367
Lee, C., Mitsialis, S. A., Aslam, M., Vitali, S. H., Vergadi, E., Konstantinou, G., Sdrimas, K., Fernandez-Gonzalez, A., & Kourembanas, S. (2012). Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation., 126(22), 2601–2611. https://doi.org/10.1161/CIRCULATIONAHA.112.114173
Zhang, B., Yin, Y., Lai, R. C., Tan, S. S., Choo, A. B., & Lim, S. K. (2014). Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev., 23(11), 1233–1244. https://doi.org/10.1089/scd.2013.0479
Dostert, G., Mesure, B., Menu, P., & Velot, É. (2017). How do mesenchymal stem cells influence or are influenced by microenvironment through extracellular vesicles communication? Front Cell Dev Biol., 5, 6–6. https://doi.org/10.3389/fcell.2017.00006
Raposo, G., & Stoorvogel, W. (2013). Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol., 200(4), 373–383. https://doi.org/10.1083/jcb.201211138
Lai, R. C., Tan, S. S., Yeo, R. W. Y., Choo, A. B. H., Reiner, A. T., Su, Y., Shen, Y., Fu, Z., Alexander, L., Sze, S. K., & Lim, S. K. (2016). MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J Extracell Ves., 5, 29828–29828. https://doi.org/10.3402/jev.v5.29828
Liang, Y., Duan, L., Lu, J., Xia, J. (2021). Engineering exosomes for targeted drug delivery. Theranostics, Vol. 11, Issue 7. https://doi.org/10.7150/thno.52570
Shurtleff, M.J., Temoche-Diaz, M.M., Karfilis, K.V., Ri, S., Schekman, R. (2016) Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. eLife;5:e19276. https://doi.org/10.7554/eLife.19276
Yokoi, A., Villar-Prados, A., Oliphint, P.A., Zhang, J., Song, X., DeHoff, P., Morey, R., Liu, J., Roszik, J., Clise-Dwyer, K., Burks, J.K., O’Halloran, T.J., Laurent, L.C., Sood, A.K. (2019). Mechanisms of nuclear content loading to exosomes, Sci Adv; 5: eaax8849 20 November 2019
Sharma, S., Mishra, R., Bigham, G. E., Wehman, B., Khan, M. M., Huichun, X., Saha, P., Goo, Y. A., Datla, S. R., Chen, L., Tulapurkar, M. E., Taylor, B. S., Yang, P., Karathanasis, S., Goodlett, D. R., & Kaushal, S. (2017). A Deep Proteome Analysis Identifies the Complete Secretome as the Functional Unit of Human Cardiac Progenitor Cells. Circ Res., 120(5), 816–834. https://doi.org/10.1161/CIRCRESAHA.116.309782
Gnecchi, M., He, H., Liang, O. D., Melo, L. G., Morello, F., Mu, H., Noiseux, N., Zhang, L., Pratt, R. E., Ingwall, J. S., & Dzau, V. J. (2005). Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med., 11, 367–368. https://doi.org/10.1038/nm0405-367
Yoon, Y. S., Wecker, A., Heyd, L., Park, J. S., Tkebuchava, T., Kusano, K., Hanley, A., Scadova, H., Qin, G., Cha, D. H., Johnson, K. L., Aikawa, R., Asahara, T., & Losordo, D. W. (2005). Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest., 115, 326–338. https://doi.org/10.1172/JCI22326
Nagaya, N., Kangawa, K., Itoh, T., Iwase, T., Murakami, S., Miyahara, Y., Fujii, T., Uematsu, M., Ohgushi, H., Yamagishi, M., Tokudome, T., Mori, H., Miyatake, K., & Kitamura, S. (2005). Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation., 112, 1128–1135. https://doi.org/10.1161/CIRCULATIONAHA.104.500447
Gnecchi, M., He, H., Noiseux, N., Liang, O. D., Zhang, L., Morello, F., Mu, H., Melo, L. G., Pratt, R. E., Ingwall, J. S., & Dzau, V. J. (2006). Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J., 20, 661–669. https://doi.org/10.1096/fj.05-5211com
Lim, S. Y., Kim, Y. S., Ahn, Y., Jeong, M. H., Hong, M. H., Joo, S. Y., Nam, K. I., Cho, J. G., Kang, P. M., & Park, J. C. (2006). The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc Res., 70, 530–542. https://doi.org/10.1016/j.cardiores.2006.02.016
Takahashi, M., Li, T. S., Suzuki, R., Kobayashi, T., Ito, H., Ikeda, Y., Matsuzaki, M., & Hamano, K. (2006). Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol., 291, H886–H893. https://doi.org/10.1152/ajpheart.00142.2006
Uemura, R., Xu, M., Ahmad, N., & Ashraf, M. (2006). Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res., 98, 1414–1421. https://doi.org/10.1161/01.RES.0000225952.61196.39
Xu, M., Uemura, R., Dai, Y., Wang, Y., Pasha, Z., & Ashraf, M. (2007). In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Mol Cell Cardiol., 42, 441–448. https://doi.org/10.1016/j.yjmcc.2006.10.009
Caplice, N. M., & Deb, A. (2004). Myocardial-cell replacement: the science, the clinic and the future. Nat Clin Pract Cardiovasc Med., 1, 90–95.
Dimmeler, S., Zeiher, A. M., & Schneider, M. D. (2005). Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest., 115, 572–583.
Melo, L. G., Pachori, A. S., Kong, D., Gnecchi, M., Wang, K., Pratt, R. E., & Dzau, V. J. (2004). Molecular and cell-based therapies for protection, rescue, and repair of ischemic myocardium: reasons for cautious optimism. Circulation., 109, 2386–2393.
Mirotsou, M., Zhang, Z., Deb, A., Zhang, L., Gnecchi, M., Noiseux, N., Mu, H., Pachori, A., & Dzau, V. (2007). Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A., 104, 1643–1648.
Colombo, M., Moita, C., van Niel, G., Kowal, J., Vigneron, J., Benaroch, P., Manel, N., Moita, L.F., Clotilde and Grac¸a Raposo. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. Journal of Cell Science 126, 5553–5565. https://doi.org/10.1242/jcs.128868
Yingjie Wang, Lan Zhang, Yongjun Li, Lijuan Chen , Xiaolong Wang, Wei Guo a, Xue Zhang b,f , Gangjian Qin d , Sheng-hu He e , Arthur Zimmerman f , Yutao Liu f , Il-man Kim f, Neal L. Weintraub f , Yaoliang Tang f. (2015) Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. International Journal of Cardiology 192 61–69, https://doi.org/10.1016/j.ijcard.2015.05.020.
Wang, J., Faict, S., Maes, K., De Bruyne, E., Van Valckenborgh, E., Schots, R., Vanderkerken, K., & Menu, E. (2016). Extracellular vesicle cross-talk in the bone marrow microenvironment: implications in multiple myeloma. Oncotarget., 7(25), 38927–38945. https://doi.org/10.18632/oncotarget.7792
Shanmuganathan, M., Vughs, J., Noseda, M., & Emanueli, C. Exosomes: Basic Biology and Technological Advancements Suggesting Their Potential as Ischemic Heart Disease Therapeutics. Front Physiol, 9, 1159. https://doi.org/10.3389/fphys.2018.01159
Peinado, H., Alecˇković, M., Lavotshkin, S., Matei, I., Costa-Silva, B., Moreno-Bueno, G., Hergueta-Redondo, M., Williams, C., García-Santos, G., Ghajar, C. M., Nitadori-Hoshino, A., Hoffman, C., Badal, K., Garcia, B. A., Callahan, M. K., Yuan, J., Martins, V. R., Skog, J., Kaplan, R. N., … Lyden, D. (2012). Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med, 18, 883–891. https://doi.org/10.1038/nm.2753
Wang, Y., Xiao, X., Zhang, J., Choudhury, R., Robertson, A., Li, K., Ma, M., Burge, C. B., & Wang, Z. (2013). A complex network of factors with overlapping affinities represses splicing through intronic elements. Nature Structural & Molecular Biology., 20, 36–45. https://doi.org/10.1038/nsmb.2459
Wei, W. J., Mu, S. R., Heiner, M., Fu, X., Cao, L. J., Gong, X. F., Bindereif, A., & Hui, J. (2012). YB-1 binds to CAUC motifs and stimulates exon inclusion by enhancing the recruitment of U2AF to weak polypyrimidine tracts. Nucleic Acids Research., 40, 8622–8636. https://doi.org/10.1093/nar/gks579
Lyabin, D. N., Eliseeva, I. A., & Ovchinnikov, L. P. (2014). YB-1 protein: functions and regulation. Wiley Interdisciplinary Reviews., 5, 95–110. https://doi.org/10.1002/wrna.1200
Asahara, T., Murohara, T., Sullivan, A., Silver, M., van der Zee, R., Li, T., Witzenbichler, B., Schatteman, G., & Isner, J. M. (1997). Isolation of putative progenitor endothelial cells for angiogenesis. Science., 275, 964–967.
Orlic, D., Kajstura, J., Chimenti, S., Jakoniuk, I., Anderson, S. M., Li, B., Pickel, J., McKay, R., Nadal-Ginard, B., Bodine, D. M., Leri, A., & Anversa, P. (2001). Bone marrow cells regenerate infarcted myocardium. Nature., 410, 701–705. https://doi.org/10.1038/35070587
Tomita, S., Li, R. K., Weisel, R. D., Mickle, D. A., Kim, E. J., Sakai, T., & Jia, Z. Q. (1999). Autologous transplantation of bone marrow cells improves damaged heart function. Circulation., 100, II247–II256.
Gnecchi, M., Zhang, Z., Ni, A., & Dzau, V. J. (2008). Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res., 103, 1204–1219. https://doi.org/10.1161/CIRCRESAHA.108.176826
Balsam, L. B., Wagers, A. J., Christensen, J. L., Kofidis, T., Weissman, I. L., & Robbins, R. C. (2004). Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature., 428, 668–673. https://doi.org/10.1038/nature02460
Murry, C. E., Soonpaa, M. H., Reinecke, H., Nakajima, H., Nakajima, H. O., Rubart, M., Pasumarthi, K. B., Virag, J. I., Bartelmez, S. H., Poppa, V., Bradford, G., Dowell, J. D., Williams, D. A., & Field, L. J. (2004). Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature., 428, 664–668. https://doi.org/10.1038/nature02446
Noiseux, N., Gnecchi, M., Lopez-Ilasaca, M., Zhang, L., Solomon, S. D., Deb, A., Dzau, V. J., & Pratt, R. E. (2006). Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther., 14, 840–850. https://doi.org/10.1016/j.ymthe.2006.05.016
Hurley, J. H. (2015). ESCRTs are everywhere. The EMBO Journal, 34, 2398–2407. https://doi.org/10.15252/embj.201592484
Roxrud, I., Stenmark, H., & Malerød, L. (2010). ESCRT & Co. Biol. Cell, 102, 293–318. https://doi.org/10.1042/BC20090161
Hurley, J. H., & Hanson, P. I. (2010). Membrane budding and scission by the ESCRT machinery: it's all in the neck. Nat. Rev. Mol. Cell Biol., 11, 556–566. https://doi.org/10.1038/nrm2937
Wollert, T., Wunder, C., Lippincott-Schwartz, J., & Hurley, J. H. (2009). Membrane Scission by the ESCRT-III Complex. Nature., 458(7235), 172–177. https://doi.org/10.1038/nature07836
Filimonenko, M., Stuffers, S., Raiborg, C., Yamamoto, A., Malerød, L., Fisher, E. M. C., Isaacs, A., Brech, A., Stenmark, H., & Simonsen, A. (2007). Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J Cell Biol, 179(3), 485–500. https://doi.org/10.1083/jcb.200702115
Stuffers, S., Wegner, C. S., Stenmark, H., & Brech, A. (2009). Multivesicular endosome biogenesis in the absence of ESCRTS. Traffic, 10, 925–937. https://doi.org/10.1111/j.1600-0854.2009.00920.x
van Niel, G., Charrin, S., Simoes, S., Romao, M., Rochin, L., Saftig, P., Marks, M. S., Rubinstein, E., & Raposo, G. (2011). The tetraspanin CD63 regulates ESCRT-independent and dependent endosomal sorting during melanogenesis. Dev Cell., 21(4), 708–721. https://doi.org/10.1016/j.devcel.2011.08.019
Bobrie, A., Colombo, M., Raposo, G., & Thery, A. C. (2011). Exosome secretion: molecular mechanisms and rolesin immune responses. Traffic, 12, 1659–1668. https://doi.org/10.1111/j.1600-0854.2011.01225.x
Gnecchi, M., Zhang, Z., Ni, A., & Dzau, V. J. (2008). Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy. Circulation Research., 103, 1204–1219. https://doi.org/10.1161/CIRCRESAHA.108.176826
Maia, J., Caja, S., Strano Moraes, M. C., Couto, N., & Costa-Silva, B. (2018). Exosome-based cell-cell communication in the tumor microenvironment. Front Cell Dev Biol., 6, 18.
Krämer-Albers, E.-M., & Hill, A. F. (2016). Extracellular vesicles: interneural shuttles of complex messages. Curr Opin Neurobiol., 39, 101–107.
Crescitelli, R., Lässer, C., Szabó, T. G., Kittel, A., Eldh, M., Dianzani, I., Buzás, E. I., & Lötvall, J. (2013). Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles., 2, 20677.
Ludwig, A. K., & Giebel, B. (2012). Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol., 44, 11–15.
Garcia, N. A., Moncayo-Arlandi, J., Sepulveda, P., & Diez-Juan, A. (2016). Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes. Cardiovasc Res., 109, 397–408.
Stoorvogel, W. (2015). Resolving sorting mechanisms into exosomes. Cell Research, (25), 531–532. https://doi.org/10.1038/cr.2015.39 published online 31 March 2015.
Colombo, M., Moita, C., van Niel, G., Kowal, J., Vigneron, J., Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. Journal of Cell Science 126, (24). https://doi.org/10.1242/jcs.128868.
van Niel, G., D'Angelo, G., & Raposo, G. (2018). Shedding light on the cell biology of extracellular vesicles. Nature Reviews Molecular Cell Biology, 19, 213–228. https://doi.org/10.1038/nrm.2017.125
Perez-Hernandez, D., Gutiérrez-Vázquez, C., Jorge, I., López-Martín, S., Ursa, A., Sánchez-Madrid, F., Vázquez, J., & Yáñez-Mó, M. (2013). The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J Biol Chem, 288(17), 11649–11661. https://doi.org/10.1074/jbc.M112.445304 Epub 2013 Mar 5.
Funding
This research was funded by National institute of Health under RO1 grant (7RO1HL141922-04) to Dr. Sunjay Kaushal.
Author information
Authors and Affiliations
Contributions
SAG: Designed and performed experiments, analyzed and interpreted data, wrote manuscript original draft and final manuscript approval. PS: helped in experimentation, data analysis and collection. LC: Performed all animal surgeries. AT: Helped in mouse heart sectioning and staining. ZDG, Supervised and helped trouble shoot, manuscript editing, JB: helped in mouse colony and data maintenance, LS: Helped in heart sectioning and staining, AS: Helped in cell culture and manuscript editing, AB: Cell culture and data maintenance, VM: Manuscript editing and mouse colony maintenance, RM: Helped in data analysis and manuscript review, SS: Supervision in data analysis and manuscript revision, AI: performed the Insilco analysis part for finding of HSEs in the promoter region of YBX1. SK: SK: Supervised the experimental work and edited the manuscript for critical review. The principal investigator of the study.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no conflict of interest.
Ethics approval
The study was ethically approved by ethics committee of Northwestern university and Lurie Children’s hospital, Chicago Ilinois, USA.
Consent to participate
Not applicable.
Consent for publication
All authors listed in the manuscript have given their consent for publication of the research work included in the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guru, S.A., Saha, P., Chen, L. et al. HSF-1 enhances cardioprotective potential of stem cells via exosome biogenesis and their miRNA cargo enrichment. Stem Cell Rev and Rep 19, 2038–2051 (2023). https://doi.org/10.1007/s12015-023-10565-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10565-7