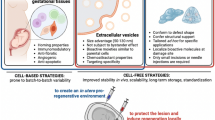
Abstract
Spina bifida is one of the neural tube defects, with a high incidence in human birth defects, which seriously affects the health and quality of life of patients. In the treatment of bone defects, the source of autologous bone is limited and will cause secondary damage to the patient. At the same time, since the bone tissue in animals needs to play a variety of biological functions, its complex structure cannot be replaced by a single material. The combination of mechanical materials and biological materials has become a common choice. Human umbilical cord mesenchymal stem cells (hUC-MSCs) have the advantages of easy access, rapid proliferation, low immunogenicity, and no ethical issues. It is often used in the clinical research of tissue regeneration and repair. Therefore, in this study, we established a spina bifida model using Japanese white rabbits. This model was used to screen the best regenerative repair products for congenital spina bifida, and to evaluate the safety of regenerative repair products. The results showed that the combination of hUC-MSCs with collagen material had better regeneration effect than collagen material alone, and had no negative impact on the health of animals. This study provides a new idea for the clinical treatment of spina bifida, and also helps to speed up the research progress of regenerative repair products.
Graphical Abstract










Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Adzick, N. S. (2010). Fetal myelomeningocele: Natural history, pathophysiology, and in-utero intervention. Seminars in Fetal and Neonatal Medicine, 15(1), 9–14.
McComb, J. G. (2015). A practical clinical classification of spinal neural tube defects. Childs Nervous System, 31(10), 1641–1657.
Tortori-Donati, P., Rossi, A., & Cama, A. (2000). Spinal dysraphism: A review of neuroradiological features with embryological correlations and proposal for a new classification. Neuroradiology, 42(7), 471–491.
Atta, C. A., et al. (2016). Global Birth Prevalence of Spina Bifida by Folic Acid Fortification Status: A Systematic Review and Meta-Analysis. American Journal of Public Health, 106(1), 24–34.
Liu, J., et al. (2016). Prevalence and trend of neural tube defects in five counties in Shanxi province of Northern China, 2000 to 2014. Birth Defects Research. Part A, Clinical and Molecular Teratology, 106(4), 267–274.
Wang, W., & Yeung, K. (2017). Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater, 2(4), 224–247.
Kim, S. Y., et al. (2012). An early comparative analysis of the use of autograft versus allograft in anterior cervical discectomy and fusion. Korean J Spine, 9(3), 142–146.
Scheerlinck, L. M., et al. (2013). Donor site complications in bone grafting: Comparison of iliac crest, calvarial, and mandibular ramus bone. International Journal of Oral and Maxillofacial Implants, 28(1), 222–227.
Thrivikraman, G., et al. (2017). Biomaterials for Craniofacial Bone Regeneration. Dental Clinics of North America, 61(4), 835–856.
Fan, Q., et al. (2021). Ridge preservation of a novel extraction socket applying Bio-Oss(R) collagen: An experimental study in dogs. Journal of Dental Sciences, 16(3), 831–839.
Pham, P. V., Vu N. B. (2020). Off-the-shelf mesenchymal stem cells from human umbilical cord tissue can significantly improve symptoms in COVID-19 patients: An analysis of evidential relations. World Journal of Stem Cells, 12(8), 721–730.
Deng, W. S., et al. (2020). Collagen scaffold combined with human umbilical cord-mesenchymal stem cells transplantation for acute complete spinal cord injury. Neural Regeneration Research, 15(9), 1686–1700.
Qu, Q., et al. (2020). Exosomes derived from human umbilical cord mesenchymal stem cells inhibit vein graft intimal hyperplasia and accelerate reendothelialization by enhancing endothelial function. Stem Cell Research & Therapy, 11(1), 133.
Bielby, R., Jones, E., McGonagle, D. (2007). The role of mesenchymal stem cells in maintenance and repair of bone. Injury, 38(1), S26–32.
Zhang, Y., Husch, J., & van den Beucken, J. (2018). Intraoperative Construct Preparation: A Practical Route for Cell-Based Bone Regeneration. Tissue Engineering. Part B, Reviews, 24(5), 403–417.
Baranovskii, D. S., et al. (2022). Minimally Manipulated Bone Marrow-Derived Cells Can Be Used for Tissue Engineering In Situ and Simultaneous Formation of Personalized Tissue Models. Bulletin of Experimental Biology and Medicine, 173(1), 139–145.
Chen, B., et al. (2007). Homogeneous osteogenesis and bone regeneration by demineralized bone matrix loading with collagen-targeting bone morphogenetic protein-2. Biomaterials, 28(6), 1027–35.
Zhang, L., et al. (2018). Therapeutic effect of human umbilical cord-derived mesenchymal stem cells on injured rat endometrium during its chronic phase. Stem Cell Research & Therapy, 9(1), 36.
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I.,Marini, F., Krause, D., Deans, R., Keating, A., Dj, P., & Horwitz, E. (2006). Minimal criteria for defining multipotent mesenchymalstromal cells. The International Society for Cellular Therapy positionstatement. Cytotherapy, 8(4), 315–317.
Werler, M. M., Shapiro, S., Mitchell, A. A. (1993). Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA, 269(10), 1257–1261.
Finnell, R.H., et al. (2021). Gene Environment Interactions in the Etiology of Neural Tube Defects. Frontiers in Genetics, 12, 659612.
Greene, N. D., Copp, A. J. (2009) Development of the vertebrate central nervous system: formation of the neural tube. Prenatal Diagnosis, 29(4), 303–311.
El-Sherbiny, M., et al. (2020). Functional beta-cells derived from umbilical cord blood mesenchymal stem cells for curing rats with streptozotocin-induced diabetes mellitus. Singapore Medical Journal, 61(1), 39–45.
Feng, Y., et al. (2020). Effects of human umbilical cord mesenchymal stem cells derived from exosomes on migration ability of endometrial glandular epithelial cells. Molecular Medicine Reports, 22(2), 715–722.
Guo, Z., et al. (2020). Administration of umbilical cord mesenchymal stem cells in patients with severe COVID-19 pneumonia. Critical Care, 24(1), 420.
Lin, M. J., et al. (2020). Plasma membrane vesicles of human umbilical cord mesenchymal stem cells ameliorate acetaminophen-induced damage in HepG2 cells: a novel stem cell therapy. Stem Cell Research & Therapy, 11(1), 225.
Liu, A. M., et al. (2020). Human adipose tissue- and umbilical cord-derived stem cells: which is a better alternative to treat spinal cord injury? Neural Regeneration Research, 15(12), 2306–2317.
Jerkic, M., et al. (2020). Human Umbilical Cord Mesenchymal Stromal Cells Attenuate Systemic Sepsis in Part by Enhancing Peritoneal Macrophage Bacterial Killing via Heme Oxygenase-1 Induction in Rats. Anesthesiology, 132(1), 140–154.
Jia, Z., Wang, S., Liu, Q. (2020). Identification of differentially expressed genes by single-cell transcriptional profiling of umbilical cord and synovial fluid mesenchymal stem cells. Journal of Cellular and Molecular Medicine, 24(2), 1945–1957.
An, J. H., et al. (2013). Transplantation of human umbilical cord blood-derived mesenchymal stem cells or their conditioned medium prevents bone loss in ovariectomized nude mice. Tissue Engineering Part A, 19(5–6), 685–696.
Hendrijantini, N., et al. (2018). A potential therapy of human umbilical cord mesenchymal stem cells for bone regeneration on osteoporotic mandibular bone. European Journal of Dental Education, 12(3), 358–362.
Zhang, S., et al. (2016). Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage, 24(12), 2135–2140.
Tomasoni, S., et al. (2013). Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells and Development, 22(5), 772–780.
Furuta, T., et al. (2016). Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Translational Medicine, 5(12), 1620–1630.
Qin, Y., et al. (2016). Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Science and Reports, 6, 21961.
Liu, J., et al. (2013). Acellular spinal cord scaffold seeded with mesenchymal stem cells promotes long-distance axon regeneration and functional recovery in spinal cord injured rats. Journal of the Neurological Sciences, 25(1–2), 127–136.
Bamba, Y., et al. (2017). Generation of Induced Pluripotent Stem Cells and Neural Stem/Progenitor Cells from Newborns with Spina Bifida Aperta. Asian Spine J, 11(6), 870–879.
Acknowledgements
The authors are very grateful to the animal experiment center of the National Research Institute for Family Planning for its meticulous care of animals.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Key Research and Development Program of China [2016YFC1000803].
Author information
Authors and Affiliations
Contributions
XCS, XM and HFX designed the study. HW, XCSand JHL were responsible for the vivo surgery. XCS and HW were responsible for in vitro experiments. HW,XCS and HFX prepared the manuscript. YFY and LQY provided the bone repair materials and collagen membranes. HW, XCS, JHL and HFX were responsible for revising the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competinginterests.
Ethical Approval
Ethical approval to report this case was obtained fromthe National Research Institute for Family Planning (Ethics Number2015-16).
Consent for Publication
All authors gave consent for publication.
Informed Consent
There are no human subjects in this article andinformed consent is not applicable.
Research involving Human and Animal Rights
All procedures in thisstudy were conducted in accordance with the National Research Institutefor Family Planning (Ethics Number 2015–16) approved protocols.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Sun, XC., Li, JH. et al. Combining Bone Collagen Material with hUC-MSCs for Applicationto Spina Bifida in a Rabbit Model. Stem Cell Rev and Rep 19, 1034–1050 (2023). https://doi.org/10.1007/s12015-022-10478-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10478-x