Abstract
The induction of feto-maternal tolerance, fetal non-immunogenicity, and the regulation of mother’s immune system are essential variables in a successful pregnancy. Fetal membranes have been used as a source of stem cells and biological components in recent decades. Human amniotic epithelial cells (hAEC) have stem/progenitor characteristics like those found in the amniotic membrane. Based on their immunomodulatory capabilities, recent studies have focused on the experimental and therapeutic applications of hAECs in allograft transplantation, autoimmune disorders, and gynecological problems such as recurrent spontaneous abortion (RSA), recurrent implantation failure (RIF), and premature ovarian failure (POF). This review discusses some of the immunomodulatory features and therapeutic potential of hAECs in preventing infertility, miscarriage, and implantation failure by controlling the maternal immune system.
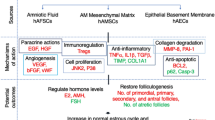
Graphical Abstract




Similar content being viewed by others
Data Availability
Not Applicable.
References
Bourne, G. (1962). The foetal membranes. A review of the anatomy of normal amnion and chorion and some aspects of their function. Postgraduate Medical Journal, 38, 193–201
Than, N. G., et al. (2019). Editorial: Fetal-Maternal Immune Interactions in Pregnancy. Frontiers In Immunology, 10, 2729
Alviano, F., et al. (2007). Term Amniotic membrane is a high throughput source for multipotent Mesenchymal Stem Cells with the ability to differentiate into endothelial cells in vitro. Bmc Developmental Biology, 7, 11
Sharma, A., & Yadav, K. (2015). Amniotic membrane - A Novel material for the root coverage: A case series. J Indian Soc Periodontol, 19(4), 444–448
Mehats, C., et al. (2011). [Biochemistry of fetal membranes rupture]. Gynécologie, Obstétrique & Fertilité, 39(6), 365–369
Mermet, I., et al. (2007). Use of amniotic membrane transplantation in the treatment of venous leg ulcers. Wound Repair And Regeneration : Official Publication Of The Wound Healing Society [And] The European Tissue Repair Society, 15(4), 459–464
Niknejad, H., et al. (2008). Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater, 15, 88–99
Ganatra, M. A. (2003). Amniotic membrane in surgery. The Journal Of The Pakistan Medical Association, 53(1), 29–32
Cai, J., et al. (2010). Generation of human induced pluripotent stem cells from umbilical cord matrix and amniotic membrane mesenchymal cells. Journal Of Biological Chemistry, 285(15), 11227–11234
Ilancheran, S., et al. (2007). Stem cells derived from human fetal membranes display multilineage differentiation potential. Biology Of Reproduction, 77(3), 577–588
Miki, T., et al. (2005). Stem cell characteristics of amniotic epithelial cells. Stem Cells, 23(10), 1549–1559
Pappa, K. I., & Anagnou, N. P. (2009). Novel sources of fetal stem cells: where do they fit on the developmental continuum? Regenerative Medicine, 4(3), 423–433
Guleria, I., & Sayegh, M. H. (2007). Maternal acceptance of the fetus: true human tolerance. The Journal Of Immunology, 178(6), 3345–3351
Moffett, A., & Loke, C. (2006). Immunology of placentation in eutherian mammals. Nature Reviews Immunology, 6(8), 584–594
Chen, S. J., Liu, Y. L., & Sytwu, H. K. (2012). Immunologic regulation in pregnancy: from mechanism to therapeutic strategy for immunomodulation. Clinical And Developmental Immunology, 2012, 258391
PrabhuDas, M., et al. (2015). Immune mechanisms at the maternal-fetal interface: perspectives and challenges. Nature Immunology, 16(4), 328–334
Kammerer, U., et al. (2008). Role of dendritic cells in the regulation of maternal immune responses to the fetus during mammalian gestation. Immunol Invest, 37(5), 499–533
Chaouat, G., et al. (2004). TH1/TH2 paradigm in pregnancy: paradigm lost? Cytokines in pregnancy/early abortion: reexamining the TH1/TH2 paradigm. International Archives Of Allergy And Immunology, 134(2), 93–119
Gardner, L., & Moffett, A. (2003). Dendritic cells in the human decidua. Biology Of Reproduction, 69(4), 1438–1446
Hanna, J., & Mandelboim, O. (2007). When killers become helpers. Trends In Immunology, 28(5), 201–206
Houser, B. L., et al. (2011). Two unique human decidual macrophage populations. The Journal Of Immunology, 186(4), 2633–2642
Liu, S., et al. (2017). The role of decidual immune cells on human pregnancy. Journal Of Reproductive Immunology, 124, 44–53
Svensson-Arvelund, J., et al. (2015). The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. The Journal Of Immunology, 194(4), 1534–1544
Moore, K. W., et al. (2001). Interleukin-10 and the interleukin-10 receptor. Annual Review Of Immunology, 19, 683–765
Mellor, A. L., et al. (2001). Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nature Immunology, 2(1), 64–68
Gonzalez, A., et al. (2012). The immunosuppressive molecule HLA-G and its clinical implications. Critical Reviews In Clinical Laboratory Sciences, 49(3), 63–84
Contini, P., et al. (2020). HLA-G Expressing Immune Cells in Immune Mediated Diseases. Frontiers In Immunology, 11, 1613
Kubo, M., et al. (2001). Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci, 42(7), 1539–1546
Tabatabaei, M., et al. (2014). Isolation and partial characterization of human amniotic epithelial cells: the effect of trypsin. Avicenna J Med Biotechnol, 6(1), 10–20
Hall, O. J., & Klein, S. L. (2017). Progesterone-based compounds affect immune responses and susceptibility to infections at diverse mucosal sites. Mucosal Immunology, 10(5), 1097–1107
Raghupathy, R., & Szekeres-Bartho, J. (2022). Progesterone: A Unique Hormone with Immunomodulatory Roles in Pregnancy.Int J Mol Sci, 23(3)
Aluvihare, V. R., Kallikourdis, M., & Betz, A. G. (2004). Regulatory T cells mediate maternal tolerance to the fetus. Nature Immunology, 5(3), 266–271
Beagley, K. W., & Gockel, C. M. (2003). Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. Fems Immunology And Medical Microbiology, 38(1), 13–22
Miyaura, H., & Iwata, M. (2002). Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. The Journal Of Immunology, 168(3), 1087–1094
Clark, D. A., Chaouat, G., & Gorczynski, R. M. (2002). Thinking outside the box: mechanisms of environmental selective pressures on the outcome of the materno-fetal relationship. American Journal Of Reproductive Immunology, 47(5), 275–282
Saito, S., et al. (1999). Distribution of Th1, Th2, and Th0 and the Th1/Th2 cell ratios in human peripheral and endometrial T cells. Am J Reprod Immunol, 42(4): p. 240-5
Rebar, R. W. (2009). Premature ovarian failure. Obstetrics And Gynecology, 113(6), 1355–1363
Dragojevic-Dikic, S., et al. (2010). An immunological insight into premature ovarian failure (POF). Autoimmun Rev, 9(11), 771–774
Hoek, A., Schoemaker, J., & Drexhage, H. A. (1997). Premature ovarian failure and ovarian autoimmunity. Endocrine Reviews, 18(1), 107–134
Chernyshov, V. P., et al. (2001). Immune disorders in women with premature ovarian failure in initial period. American Journal Of Reproductive Immunology, 46(3), 220–225
Tung, K. S., et al. (2001). Autoimmune ovarian disease: mechanism of induction and prevention. J Soc Gynecol Investig, 8(1 Suppl Proceedings): p. S49-51
Corenblum, B., Rowe, T., & Taylor, P. J. (1993). High-dose, short-term glucocorticoids for the treatment of infertility resulting from premature ovarian failure. Fertility And Sterility, 59(5), 988–991
Sheikhansari, G., et al. (2018). Current approaches for the treatment of premature ovarian failure with stem cell therapy. Biomedicine & Pharmacotherapy, 102, 254–262
Wang, J., et al. (2021). Research Progress on the Treatment of Premature Ovarian Failure Using Mesenchymal Stem Cells: A Literature Review. Front Cell Dev Biol, 9, 749822
Goswami, D., & Conway, G. S. (2005). Premature ovarian failure. Human Reproduction Update, 11(4), 391–410
Kalu, E., & Panay, N. (2008). Spontaneous premature ovarian failure: management challenges. Gynecological Endocrinology, 24(5), 273–279
Coughlan, C., et al. (2014). Recurrent implantation failure: definition and management. Reproductive Biomedicine Online, 28(1), 14–38
Orvieto, R., Brengauz, M., & Feldman, B. (2015). A novel approach to normal responder patient with repeated implantation failures–a case report. Gynecological Endocrinology, 31(6), 435–437
Moffett, A., & Shreeve, N. (2015). First do no harm: uterine natural killer (NK) cells in assisted reproduction. Human Reproduction, 30(7), 1519–1525
Male, V., et al. (2010). Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. The Journal Of Immunology, 185(7), 3913–3918
Seshadri, S., & Sunkara, S. K. (2014). Natural killer cells in female infertility and recurrent miscarriage: a systematic review and meta-analysis. Human Reproduction Update, 20(3), 429–438
Kwak-Kim, J. Y., et al. (2003). Increased T helper 1 cytokine responses by circulating T cells are present in women with recurrent pregnancy losses and in infertile women with multiple implantation failures after IVF. Human Reproduction, 18(4), 767–773
Geva, E., et al. (2000). Prednisone and aspirin improve pregnancy rate in patients with reproductive failure and autoimmune antibodies: a prospective study. American Journal Of Reproductive Immunology, 43(1), 36–40
Azem, F., et al. (2004). Increased rates of thrombophilia in women with repeated IVF failures. Human Reproduction, 19(2), 368–370
Achache, H., et al. (2010). Defective endometrial prostaglandin synthesis identified in patients with repeated implantation failure undergoing in vitro fertilization. Fertility And Sterility, 94(4), 1271–1278
Chakraborty, I., Das, S. K., & Dey, S. K. (1995). Differential expression of vascular endothelial growth factor and its receptor mRNAs in the mouse uterus around the time of implantation. Journal Of Endocrinology, 147(2), 339–352
Folkman, J. (1995). Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Medicine, 1(1), 27–31
Halder, J. B., et al. (2000). Differential expression of VEGF isoforms and VEGF(164)-specific receptor neuropilin-1 in the mouse uterus suggests a role for VEGF(164) in vascular permeability and angiogenesis during implantation. Genesis, 26(3), 213–224
Hyder, S. M., et al. (2000). Identification of functional estrogen response elements in the gene coding for the potent angiogenic factor vascular endothelial growth factor. Cancer Research, 60(12), 3183–3190
Chung, I. B., et al. (2000). Expression and regulation of vascular endothelial growth factor in a first trimester trophoblast cell line. Placenta, 21(4), 320–324
Qian, D., et al. (2004). Involvement of ERK1/2 pathway in TGF-beta1-induced VEGF secretion in normal human cytotrophoblast cells. Molecular Reproduction And Development, 68(2), 198–204
Ferrara, N., & Davis-Smyth, T. (1997). The biology of vascular endothelial growth factor. Endocrine Reviews, 18(1), 4–25
Zwierzchowska, A., et al. (2018). Recurrent miscarriage is associated with increased ghrelin mRNA expression in the endometrium- a case-control study. Reproductive Biology, 18(1), 12–17
Karim, S., et al. (2017). Genomic answers for recurrent spontaneous abortion in Saudi Arabia: An array comparative genomic hybridization approach. Reproductive Biology, 17(2), 133–143
Diejomaoh, M. F. (2015). Recurrent spontaneous miscarriage is still a challenging diagnostic and therapeutic quagmire. Medical Principles And Practice : International Journal Of The Kuwait University, Health Science Centre, 24(Suppl 1), 38–55
Saifi, B., et al. (2014). Th17 cells and related cytokines in unexplained recurrent spontaneous miscarriage at the implantation window. Reproductive Biomedicine Online, 29(4), 481–489
Sasaki, T., et al. (1997). Increased frequency of HLA-DR4 allele in women with unexplained recurrent spontaneous abortions, detected by the method of PCR-SSP. Journal Of Reproductive Immunology, 32(3), 273–279
Yang, H., et al. (2009). Proportional change of CD4 + CD25 + regulatory T cells after lymphocyte therapy in unexplained recurrent spontaneous abortion patients. Fertility And Sterility, 92(1), 301–305
Trundley, A., & Moffett, A. (2004). Human uterine leukocytes and pregnancy. Tissue Antigens, 63(1), 1–12
Fedorcsak, P., et al. (2004). Impact of overweight and underweight on assisted reproduction treatment. Human Reproduction, 19(11), 2523–2528
McQueen, D. B., et al. (2015). Pregnancy outcomes in women with chronic endometritis and recurrent pregnancy loss. Fertility And Sterility, 104(4), 927–931
Gilman-Sachs, A., et al. (1999). Natural killer (NK) cell subsets and NK cell cytotoxicity in women with histories of recurrent spontaneous abortions. American Journal Of Reproductive Immunology, 41(1), 99–105
Ohta, A., et al. (2012). The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Frontiers In Immunology, 3, 190
Quenby, S., et al. (1999). Pre-implantation endometrial leukocytes in women with recurrent miscarriage. Human Reproduction, 14(9), 2386–2391
Yuan, J., et al. (2015). Characterization of the subsets of human NKT-like cells and the expression of Th1/Th2 cytokines in patients with unexplained recurrent spontaneous abortion. Journal Of Reproductive Immunology, 110, 81–88
Zhu, L., et al. (2017). Treg/Th17 Cell Imbalance and IL-6 Profile in Patients With Unexplained Recurrent Spontaneous Abortion. Reprod Sci, 24(6), 882–890
Fu, B., Tian, Z., & Wei, H. (2014). TH17 cells in human recurrent pregnancy loss and pre-eclampsia. Cellular & Molecular Immunology, 11(6), 564–570
Wang, W. J., et al. (2010). Increased prevalence of T helper 17 (Th17) cells in peripheral blood and decidua in unexplained recurrent spontaneous abortion patients. Journal Of Reproductive Immunology, 84(2), 164–170
Trussell, J., et al. (2009). Cost effectiveness of contraceptives in the United States. Contraception, 79(1), 5–14
Said, E. A., et al. (2010). Programmed death-1-induced interleukin-10 production by monocytes impairs CD4 + T cell activation during HIV infection. Nature Medicine, 16(4), 452–459
Alijotas-Reig, J., Llurba, E., & Gris, J. M. (2014). Potentiating maternal immune tolerance in pregnancy: a new challenging role for regulatory T cells. Placenta, 35(4), 241–248
Yang, D., et al. (2021). Role of Transforming Growth Factor-beta1 in Regulating Fetal-Maternal Immune Tolerance in Normal and Pathological Pregnancy. Frontiers In Immunology, 12, 689181
Parhizkar, F., et al. (2021). The Impact of New Immunological Therapeutic Strategies on Recurrent Miscarriage and Recurrent Implantation Failure. Immunology Letters, 236, 20–30
Carp, H. (2019). Immunotherapy for recurrent pregnancy loss. Best Pract Res Clin Obstet Gynaecol, 60, 77–86
Deng, T., Liao, X., & Zhu, S. (2022). Recent Advances in Treatment of Recurrent Spontaneous Abortion. Obstetrical & Gynecological Survey, 77(6), 355–366
Francisco, P. D., Tan-Lim, C. S. C., Agcaoili-De, M. S. L., & Jesus (2022). Efficacy of lymphocyte immunotherapy in the treatment of recurrent pregnancy loss from alloimmunity: A systematic review and meta-analysis.Am J Reprod Immunol, : p.e13605
Jeve, Y. B., & Davies, W. (2014). Evidence-based management of recurrent miscarriages. J Hum Reprod Sci, 7(3), 159–169
Miki, T. (2018). Stem cell characteristics and the therapeutic potential of amniotic epithelial cells. American Journal Of Reproductive Immunology, 80(4), e13003
Kim, J. S., et al. (2000). Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn. Experimental Eye Research, 70(3), 329–337
Akashi, T., et al. (1999). Synthesis of basement membrane by gastrointestinal cancer cell lines. The Journal Of Pathology, 187(2), 223–228
Iozzo, R. V., & Murdoch, A. D. (1996). Proteoglycans of the extracellular environment: clues from the gene and protein side offer novel perspectives in molecular diversity and function. The Faseb Journal, 10(5), 598–614
Li, W., et al. (2006). Amniotic membrane induces apoptosis of interferon-gamma activated macrophages in vitro. Experimental Eye Research, 82(2), 282–292
Seo, J. H., Kim, Y. H., & Kim, J. S. (2008). Properties of the amniotic membrane may be applicable in cancer therapy. Medical Hypotheses, 70(4), 812–814
He, D., et al. (2020). LOXL2 from human amniotic mesenchymal stem cells accelerates wound epithelialization by promoting differentiation and migration of keratinocytes. Aging (Albany NY), 12(13), 12960–12986
Miki, T., et al. (2007). Isolation of amniotic epithelial stem cells.Curr Protoc Stem Cell Biol, Chapter 1: p. Unit 1E 3.
Chen, Y. T., et al. (2007). Human amniotic epithelial cells as novel feeder layers for promoting ex vivo expansion of limbal epithelial progenitor cells. Stem Cells, 25(8), 1995–2005
Li, H., et al. (2005). Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci, 46(3), 900–907
McDonald, C. A., et al. (2015). Immunosuppressive potential of human amnion epithelial cells in the treatment of experimental autoimmune encephalomyelitis. J Neuroinflammation, 12, 112
Motedayyen, H., et al. (2017). Method and key points for isolation of human amniotic epithelial cells with high yield, viability and purity. Bmc Research Notes, 10(1), 552
Konofaos, P., & Terzis, J. K. (2013). FK506 and nerve regeneration: past, present, and future. Journal Of Reconstructive Microsurgery, 29(3), 141–148
Wolbank, S., et al. (2007). Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: a comparison with human mesenchymal stem cells from adipose tissue. Tissue Engineering, 13(6), 1173–1183
Samarkanova, D., et al. (2021). Cord blood and amniotic membrane extract eye drop preparations display immune-suppressive and regenerative properties. Scientific Reports, 11(1), 13754
Wu, B., et al. (2021). Conditioned Medium of Human Amniotic Epithelial Cells Alleviates Experimental Allergic Conjunctivitis Mainly by IL-1ra and IL-10. Frontiers In Immunology, 12, 774601
Liu, J., et al. (2021). Human Amniotic Epithelial Cells Promote the Proliferation of Human Corneal Endothelial Cells by Regulating Telomerase Activity via the Wnt/beta-catenin Pathway. Current Eye Research, 46(2), 159–167
Evans, M. A., et al. (2018). Acute or Delayed Systemic Administration of Human Amnion Epithelial Cells Improves Outcomes in Experimental Stroke. Stroke, 49(3), 700–709
Lim, R., et al. (2017). A Pilot Study Evaluating the Safety of Intravenously Administered Human Amnion Epithelial Cells for the Treatment of Hepatic Fibrosis. Frontiers In Pharmacology, 8, 549
Yawno, T., et al. (2017). Human Amnion Epithelial Cells Protect Against White Matter Brain Injury After Repeated Endotoxin Exposure in the Preterm Ovine Fetus. Cell Transplantation, 26(4), 541–553
Zhang, Q., et al. (2019). Human Amniotic Epithelial Cell-Derived Exosomes Restore Ovarian Function by Transferring MicroRNAs against Apoptosis. Mol Ther Nucleic Acids, 16, 407–418
Zhu, D., et al. (2017). Human amnion cells reverse acute and chronic pulmonary damage in experimental neonatal lung injury. Stem Cell Research & Therapy, 8(1), 257
Di Germanio, C., et al. (2016). Amniotic Epithelial Cells: A New Tool to Combat Aging and Age-Related Diseases? Front Cell Dev Biol, 4, 135
Hodge, A., et al. (2014). Soluble factors derived from human amniotic epithelial cells suppress collagen production in human hepatic stellate cells. Cytotherapy, 16(8), 1132–1144
Basu, J., & Ludlow, J. W. (2016). Exosomes for repair, regeneration and rejuvenation. Expert Opinion On Biological Therapy, 16(4), 489–506
Maguire, G. (2013). Stem cell therapy without the cells. Communicative & Integrative Biology, 6(6), e26631
Singla, D. K. (2016). Stem cells and exosomes in cardiac repair. Current Opinion In Pharmacology, 27, 19–23
Yao, X., et al. (2016). The Paracrine Effect of Transplanted Human Amniotic Epithelial Cells on Ovarian Function Improvement in a Mouse Model of Chemotherapy-Induced Primary Ovarian Insufficiency. Stem Cells Int, 2016: p. 4148923
Kamiya, K., et al. (2005). Topical application of culture supernatant from human amniotic epithelial cells suppresses inflammatory reactions in cornea. Experimental Eye Research, 80(5), 671–679
Kim, T. H., et al. (2013). Effects of conditioned media from human amniotic epithelial cells on corneal alkali injuries in rabbits. Journal Of Veterinary Science, 14(1), 61–67
Zhang, Q., et al. (2015). Human amniotic epithelial cells inhibit granulosa cell apoptosis induced by chemotherapy and restore the fertility. Stem Cell Research & Therapy, 6, 152
Tan, J. L., et al. (2014). Human amnion epithelial cells mediate lung repair by directly modulating macrophage recruitment and polarization. Cell Transplantation, 23(3), 319–328
Zhang, Q., et al. (2017). Paracrine effects of human amniotic epithelial cells protect against chemotherapy-induced ovarian damage. Stem Cell Research & Therapy, 8(1), 270
Wang, F., et al. (2013). Human amniotic epithelial cells can differentiate into granulosa cells and restore folliculogenesis in a mouse model of chemotherapy-induced premature ovarian failure. Stem Cell Research & Therapy, 4(5), 124
Zhao, B., et al. (2017). Exosomes derived from human amniotic epithelial cells accelerate wound healing and inhibit scar formation. Journal Of Molecular Histology, 48(2), 121–132
Zhao, B., et al. (2017). [Effects of human amniotic epithelial stem cells-derived exosomes on healing of wound with full-thickness skin defect in rats]. Zhonghua Shao Shang Za Zhi, 33(1), 18–23
Zhao, B., et al. (2018). Exosomal MicroRNAs Derived from Human Amniotic Epithelial Cells Accelerate Wound Healing by Promoting the Proliferation and Migration of Fibroblasts. Stem Cells Int, 2018: p. 5420463
Zhang, Q., & Lai, D. (2020). Application of human amniotic epithelial cells in regenerative medicine: a systematic review. Stem Cell Research & Therapy, 11(1), 439
Kuk, N., et al. (2019). Human amnion epithelial cells and their soluble factors reduce liver fibrosis in murine non-alcoholic steatohepatitis. Journal Of Gastroenterology And Hepatology, 34(8), 1441–1449
Xiao, G. Y., et al. (2016). Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Scientific Reports, 6, 23120
Ding, C., et al. (2017). Different therapeutic effects of cells derived from human amniotic membrane on premature ovarian aging depend on distinct cellular biological characteristics. Stem Cell Research & Therapy, 8(1), 173
Ding, C., et al. (2018). Human amniotic mesenchymal stem cells improve ovarian function in natural aging through secreting hepatocyte growth factor and epidermal growth factor. Stem Cell Research & Therapy, 9(1), 55
Araujo, A. B., et al. (2017). Comparison of human mesenchymal stromal cells from four neonatal tissues: Amniotic membrane, chorionic membrane, placental decidua and umbilical cord. Cytotherapy, 19(5), 577–585
Toda, A., et al. (2007). The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. Journal Of Pharmacological Sciences, 105(3), 215–228
Hou, S., et al. (2020). Vitamin C improves the therapeutic potential of human amniotic epithelial cells in premature ovarian insufficiency disease. Stem Cell Research & Therapy, 11(1), 159
Khadem, F., et al. (2019). Immunoregulatory Effects of Human Amnion Epithelial Cells on Natural Killer and T Cells in Women with Recurrent Spontaneous Abortion (RSA). Turkish journal of immunology, 7(1), 21–30
Long, E. O. (1999). Regulation of immune responses through inhibitory receptors. Annual Review Of Immunology, 17, 875–904
Alter, G., Malenfant, J. M., & Altfeld, M. (2004). CD107a as a functional marker for the identification of natural killer cell activity. Journal Of Immunological Methods, 294(1–2), 15–22
Morandi, F., et al. (2019). Ectonucleotidase Expression on Human Amnion Epithelial Cells: Adenosinergic Pathways and Dichotomic Effects on Immune Effector Cell Populations. The Journal Of Immunology, 202(3), 724–735
Motedayyen, H., et al. (2018). Immunomodulatory effects of human amniotic epithelial cells on naive CD4(+) T cells from women with unexplained recurrent spontaneous abortion. Placenta, 71, 31–40
Motedayyen, H., et al. (2018). Human amniotic epithelial cells inhibit activation and pro-inflammatory cytokines production of naive CD4 + T cells from women with unexplained recurrent spontaneous abortion. Reproductive Biology, 18(2), 182–188
Liu, Y. H., et al. (2012). Amniotic epithelial cells from the human placenta potently suppress a mouse model of multiple sclerosis. PLoS One, 7(4), e35758
Khan, I., et al. (2015). Effects of Wharton’s jelly-derived mesenchymal stem cells on neonatal neutrophils. J Inflamm Res, 8, 1–8
Motedayyen, H., et al. (2018). The effect of lipopolysaccharide on anti-inflammatory and pro-inflammatory cytokines production of human amniotic epithelial cells. Reproductive Biology, 18(4), 404–409
Alipour, R., et al. (2020). Human Amniotic Epithelial Cells Affect the Functions of Neutrophils. Int J Stem Cells, 13(2), 212–220
Taheri, R. A., et al. (2018). The effect of lipopolysaccharide on the expression level of immunomodulatory and immunostimulatory factors of human amniotic epithelial cells. Bmc Research Notes, 11(1), 343
Gonzalez, C. R., et al. (2010). Expression of the TGF-beta1 system in human testicular pathologies. Reproductive Biology And Endocrinology : Rb&E, 8, 148
Letterio, J. J., & Roberts, A. B. (1998). Regulation of immune responses by TGF-beta. Annual Review Of Immunology, 16, 137–161
Alhomrani, M., et al. (2017). The Human Amnion Epithelial Cell Secretome Decreases Hepatic Fibrosis in Mice with Chronic Liver Fibrosis. Frontiers In Pharmacology, 8, 748
Lim, R., et al. (2013). Preterm human amnion epithelial cells have limited reparative potential. Placenta, 34(6), 486–492
Yu, D., et al. (2008). Asherman syndrome–one century later. Fertility And Sterility, 89(4), 759–779
Li, B., et al. (2019). Human amniotic epithelial cells improve fertility in an intrauterine adhesion mouse model. Stem Cell Research & Therapy, 10(1), 257
Harris, J. (2011). Autophagy and cytokines. Cytokine, 56(2), 140–144
Gan, L., et al. (2017). Human amniotic mesenchymal stromal cell transplantation improves endometrial regeneration in rodent models of intrauterine adhesions. Cytotherapy, 19(5), 603–616
Ouyang, X., et al. (2020). Transplantation of Human Amnion Epithelial Cells Improves Endometrial Regeneration in Rat Model of Intrauterine Adhesions. Stem Cells And Development, 29(20), 1346–1362
Abdo, R. J. (2016). Treatment of diabetic foot ulcers with dehydrated amniotic membrane allograft: a prospective case series. Journal Of Wound Care, 25(Sup7), S4–S9
Clare, G., et al., Amniotic membrane transplantation for acute ocular burns.Cochrane Database Syst Rev, 2012(9): p.CD009379
Xu, H., et al. (2019). Therapeutic Potential of Human Amniotic Epithelial Cells on Injuries and Disorders in the Central Nervous System. Stem Cells Int, 2019, 5432301
Hu, L., Z.C., Safety and Therapeutic Effect of Human Amniotic Epithelial Cells in Severe Refractory Asherman’s Syndrome. NCT03223454,2017–2021
Wang Liang, L. Y.,Human Amniotic Epithelial Stem Cell in Treatment of Refractory Severe Intrauterine Adhesion.NCT03381807,2020–2023
Lia, D., W.Q., A Therapeutic Trial of Human Amniotic Epithelial Cells Transplantation for Primary Ovarian Failure. NCT02912104,2016–2022
Zhang, C., Z.H., Clinical Study of Minimally Invasive Implantation of Human Amniotic Epithelial Cells in the Treatment of Premature Ovarian Insufficiency. NCT03207412,2017–2019
Akle, C. A., et al. (1981). Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet, 2(8254), 1003–1005
Adly, O. A., Abbas, A. M. M. A. H., Ellabban, A. M., Ali, O. S., & Mohamed, B. A. (2010).Assessment of amniotic and polyurethane membrane dressings in the treatment of burns. Burns, 36(5):703–710
Gramignoli, R., et al. (2016). Isolation of Human Amnion Epithelial Cells According to Current Good Manufacturing Procedures.Curr Protoc Stem Cell Biol, 37: p. 1E 10 1-1E 10 13.
Funding
This work was supported by the Isfahan University of Medical Sciences, Isfahan, Iran (grant No. 398865).
Author information
Authors and Affiliations
Contributions
F.R. and N.E. designed the study. F.R. and N.E. wrote the paper in consultation with A.R. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not Applicable.
Consent to Participate
Not Applicable.
Consent to Publish
Not Applicable.
Competing Interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rezayat, F., Esmaeil, N. & Rezaei, A. Potential Therapeutic Effects of Human Amniotic Epithelial Cells on Gynecological Disorders Leading to Infertility or Abortion. Stem Cell Rev and Rep 19, 368–381 (2023). https://doi.org/10.1007/s12015-022-10464-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10464-3