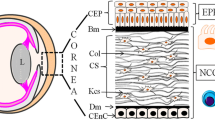
Abstract
Deficiency and dysfunction of corneal cells leads to the blindness observed in corneal diseases such as limbal stem cell deficiency (LSCD) and bullous keratopathy. Regenerative cell therapies and engineered corneal tissue are promising treatments for these diseases [1]. However, these treatments are not yet clinically feasible due to inadequate cell sources. The discovery of induced pluripotent stem cells (iPSCs) by Shinya Yamanaka has provided a multitude of opportunities in research because iPSCs can be generated from somatic cells, thus providing an autologous and unlimited source for corneal cells. Compared to other stem cell sources such as mesenchymal and embryonic, iPSCs have advantages in differentiation potential and ethical concerns, respectively. Efforts have been made to use iPSCs to model corneal disorders and diseases, drug testing [2], and regenerative medicine [1]. Autologous treatments based on iPSCs can be exorbitantly expensive and time-consuming, but development of stem cell banks with human leukocyte antigen (HLA)- homozygous cell lines can provide cost- and time-efficient allogeneic alternatives. In this review, we discuss the early development of the cornea because protocols differentiating iPSCs toward corneal lineages rely heavily upon recapitulating this development. Differentiation of iPSCs toward corneal cell phenotypes have been analyzed with an emphasis on feeder-free, xeno-free, and well-defined protocols, which have clinical relevance. The application, challenges, and potential of iPSCs in corneal research are also discussed with a focus on hurdles that prevent clinical translation.
Graphical abstract



Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- AQP1:
-
Aquaporin 1
- bFGF:
-
Basic fibroblast growth factor
- BMP:
-
Bone morphogenetic protein
- CEndoC:
-
Corneal endothelial cells
- CEpiC:
-
Corneal epithelial cells
- CGMP:
-
Current Good Manufacturing Practices
- CK:
-
Cytokeratin
- CLAU:
-
Conjunctival Limbal Autograft
- CLET:
-
Cultivated Limbal Epithelial Transplantation
- Dkk2:
-
Dickkopf WNT Signaling Pathway Inhibitor 2
- EGF:
-
Epidermal growth factor
- ESC:
-
Embryonic stem cells
- FACS:
-
Fluorescence-activated cell sorting
- FGF:
-
Fibroblast growth factor
- HLA:
-
Human leukocyte antigen
- iPSCs:
-
Induced pluripotent stem cells
- LSC:
-
Limbal stem cell
- LSCD:
-
Limbal stem cell deficiency
- MACS:
-
Magnetic cell sorting
- NCC:
-
Neural crest cells
- PDGF-BB:
-
Platelet-derived growth factor B
- SEAM:
-
Self-formed ectodermal autonomous multi-zones
- SLC4A11:
-
Solute carrier family 4-member 11
- SLC4A4:
-
Sodium bicarbonate cotransporter 1
- SLET:
-
Simple Limbal Epithelial Transplantation
- TGF-β:
-
Transforming growth factor beta
- Wnt:
-
Wingless-related integration site
- ZO-1:
-
Zonula occludens-1
References
Griffith, M., Polisetti, N., Kuffova, L., Gallar, J., Forrester, J., Vemuganti, G. K., & Fuchsluger, T. A. (2012). Regenerative approaches as alternatives to donor allografting for restoration of corneal function. The Ocular Surface, 10(3), 170–183.
Boutin, M. E., Hampton, C., Quinn, R., Ferrer, M., & Song, M. J. (2019). 3D engineering of ocular tissues for disease modeling and drug testing. In Pluripotent Stem Cells in Eye Disease Therapy (pp. 171–193). Springer. https://link.springer.com/chapter/10.1007/978-3-030-28471-8_7#citeas
Centers of Disease Control and Prevention. (n.d.). Fast Facts of Common Eye Disorders. U.S. Department of Health & Human Services. Retrieved November 9, 2021, from https://www.cdc.gov/visionhealth/basics/ced/fastfacts.htm
Whitcher, J. P., Srinivasan, M., & Upadhyay, M. P. (2001). Corneal blindness: A global perspective. Bulletin of the World Health Organization, 79, 214–221.
Gain, P., Jullienne, R., He, Z., et al. (2015). Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmology, 1342, 1–8.
Ple-Plakon, P. A., & Shtein, R. M. (2014). Trends in corneal transplantation: Indications and techniques. Current Opinion in Ophthalmology, 25(4), 300–305.
Holland, G., Pandit, A., Sánchez-Abella, L., Haiek, A., Loinaz, I., Dupin, D., ... & Ritter, T. (2021). Artificial cornea: Past, current, and future directions. Frontiers in Medicine, 8, 770780.
Mahabadi, N., Czyz, C. N., Tariq, M., & Havens, S. J. (2020). Corneal graft rejection. StatPearls Publishing.
De Araujo, A. L., & Gomes, J. Á. P. (2015). Corneal stem cells and tissue engineering: Current advances and future perspectives. World Journal of Stem Cells, 7(5), 806.
Huang, Y. X., & Li, Q. H. (2007). An active artificial cornea with the function of inducing new corneal tissue generation in vivo—A new approach to corneal tissue engineering. Biomedical Materials, 2(3), S121.
Germain, L., Carrier, P., Auger, F. A., Salesse, C., & Guérin, S. L. (2000). Can we produce a human corneal equivalent by tissue engineering? Progress in Retinal and Eye Research, 19(5), 497–527.
Kong, B., Sun, W., Chen, G., Tang, S., Li, M., Shao, Z., & Mi, S. (2017). Tissue-engineered cornea constructed with compressed collagen and laser-perforated electrospun mat. Scientific Reports, 7(1), 1–13.
Bouchard, C. (2010, February 25). Simplified surgical technique for harvesting corneal stem cells. Healio. https://www.healio.com/news/ophthalmology/20120331/simplified-surgical-technique-for-harvesting-corneal-stem-cells
Hayashi, R., Ishikawa, Y., Ito, M., et al. (2012). Generation of Corneal Epithelial Cells from Induced Pluripotent Stem Cells Derived from Human Dermal Fibroblast and Corneal Limbal Epithelium. PLoS ONE, 79, e45435.
Shalom-Feuerstein, R., Serror, L., De La Forest Divonne, S., Petit, I., Aberdam, E., Camargo, L., et al. (2012). Pluripotent stem cell model reveals essential roles for miR-450b-5p and miR-184 in embryonic corneal lineage specification. Stem Cells, 30(5), 898–909.
Ahmad, S., Stewart, R., Yung, S., Kolli, S., Armstrong, L., Stojkovic, M., et al. (2007). Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells, 25(5), 1145–1155.
Brzeszczynska, J., Samuel, K., Greenhough, S., Ramaesh, K., Dhillon, B., Hay, D. C., & Ross, J. A. (2014). Differentiation and molecular profiling of human embryonic stem cell-derived corneal epithelial cells. International Journal of Molecular Medicine, 33(6), 1597–1606.
Miesfeld, J. B., & Brown, N. L. (2019). Eye organogenesis: A hierarchical view of ocular development. Current Topics in Developmental Biology, 132, 351–393.
Graw, J. (2010). Eye development. Current Topics in Developmental Biology, 90, 343–386.
DelMonte, D. W., & Kim, T. (2011). Anatomy and physiology of the cornea. Journal of Cataract & Refractive Surgery, 37(3), 588–598.
Lwigale, P. Y. (2015). Corneal development: Different cells from a common progenitor. Progress in Molecular Biology and Translational Science, 134, 43–59.
Gage, P. J., Kuang, C., & Zacharias, A. L. (2014). The homeodomain transcription factor PITX2 is required for specifying correct cell fates and establishing angiogenic privilege in the developing cornea. Developmental Dynamics, 243(11), 1391–1400.
Zhang, J., Upadhya, D., Lu, L., & Reneker, L. W. (2015). Fibroblast growth factor receptor 2 (FGFR2) is required for corneal epithelial cell proliferation and differentiation during embryonic development. PLoS One, 10(1), e0117089.
Li, G., Xu, F., Zhu, J., Krawczyk, M., Zhang, Y., Yuan, J., Patel, S., et al. (2015). Transcription factor PAX6 (paired box 6) controls limbal stem cell lineage in development and disease. Journal of Biological Chemistry, 290(33), 20448–20454.
Collomb, E., Yang, Y., Foriel, S., Cadau, S., Pearton, D. J., & Dhouailly, D. (2013). The corneal epithelium and lens develop independently from a common pool of precursors. Developmental Dynamics, 242(5), 401–413.
Dhouailly, D., Pearton, D. J., & Michon, F. (2014). The vertebrate corneal epithelium: From early specification to constant renewal. Developmental Dynamics, 243(10), 1226–1241.
Mukhopadhyay, M., Gorivodsky, M., Shtrom, S., Grinberg, A., Niehrs, C., Morasso, M. I., & Westphal, H. (2006). Dkk2 plays an essential role in the corneal fate of the ocular surface epithelium. Development, 133(11), 2149–2154.
Rodrigues, M., Ben-Zvi, A., Krachmer, J., Schermer, A., & Sun, T. T. (1987). Suprabasal expression of a 64-kilodalton keratin (no. 3) in developing human corneal epithelium. Differentiation, 34(1), 60–67.
Gage, P. J., & Zacharias, A. L. (2009). Signaling “cross-talk” is integrated by transcription factors in the development of the anterior segment in the eye. Developmental dynamics: An official publication of the American Association of Anatomists, 238(9), 2149–2162.
Wurdak, H., Ittner, L. M., Lang, K. S., Leveen, P., Suter, U., Fischer, J. A., et al. (2005). Inactivation of TGFβ signaling in neural crest stem cells leads to multiple defects reminiscent of DiGeorge syndrome. Genes & Development, 19(5), 530–535.
Berry, F. B., Lines, M. A., Oas, J. M., Footz, T., Underhill, D. A., Gage, P. J., & Walter, M. A. (2006). Functional interactions between FOXC1 and PITX2 underlie the sensitivity to FOXC1 gene dose in Axenfeld-Rieger syndrome and anterior segment dysgenesis. Human Molecular Genetics, 15(6), 905–919.
Silla, Z. T., Naidoo, J., Kidson, S. H., & Sommer, P. (2014). Signals from the lens and Foxc1 regulate the expression of key genes during the onset of corneal endothelial development. Experimental Cell Research, 322(2), 381–388.
Matt, N., Ghyselinck, N. B., Pellerin, I., & Dupé, V. (2008). Impairing retinoic acid signalling in the neural crest cells is sufficient to alter entire eye morphogenesis. Developmental Biology, 320(1), 140–148.
Wulle, K. G. (1972). Electron microscopy of the fetal development of the corneal endothelium and Descemet’s membrane of the human eye. Investigative Ophthalmology & Visual Science, 11(11), 897–904.
Wulle, K. G., Ruprecht, K. W., & Windrath, L. C. (1974). Electron microscopy of the development of the cell junctions in the embryonic and fetal human corneal endothelium. Investigative Ophthalmology & Visual Science, 13(12), 923–934.
Bourne, W. M. (2003). Biology of the corneal endothelium in health and disease. Eye, 17(8), 912–918.
Gage, P. J., Suh, H., & Camper, S. A. (1999). Dosage requirement of Pitx2 for development of multiple organs. Development, 126(20), 4643–4651.
Seo, S., Chen, L., Liu, W., Zhao, D., Schultz, K. M., Sasman, A., et al. (2017). Foxc1 and foxc2 in the neural crest are required for ocular anterior segment development. Investigative Ophthalmology & Visual Science, 58(3), 1368–1377.
Seo, S., Singh, H. P., Lacal, P. M., Sasman, A., Fatima, A., Liu, T., et al. (2012). Forkhead box transcription factor FoxC1 preserves corneal transparency by regulating vascular growth. Proceedings of the National Academy of Sciences, 109(6), 2015–2020.
Creuzet, S., Vincent, C., & Couly, G. (2003). Neural crest derivatives in ocular and periocular structures. International Journal of Developmental Biology, 49(2–3), 161–171.
Hirsch, M., Noske, W., Prenant, G., & Renard, G. (1999). Fine structure of the developing avian corneal stroma as revealed by quick-freeze, deep-etch electron microscopy. Experimental Eye Research, 69(3), 267–277.
Doane, K. J., Ting, W. H., McLaughlin, J. S., & Birk, D. E. (1996). Spatial and temporal variations in extracellular matrix of periocular and corneal regions during corneal stromal development. Experimental Eye Research, 62(3), 285–292.
Connon, C. J., Siegler, V., Meek, K. M., Hodson, S. A., Caterson, B., Kinoshita, S., & Quantock, A. J. (2003). Proteoglycan alterations and collagen reorganisation in the secondary avian cornea during development. Ophthalmic Research, 35(4), 177–184.
Theerakittayakorn, K., Thi Nguyen, H., Musika, J., Kunkanjanawan, H., Imsoonthornruksa, S., Somredngan, S., et al. (2020). Differentiation induction of human stem cells for corneal epithelial regeneration. International Journal of Molecular Sciences, 21(21), 7834.
Ueno, H., Kurokawa, M. S., Kayama, M., Homma, R., Kumagai, Y., Masuda, C., et al. (2007). Experimental transplantation of corneal epithelium-like cells induced by Pax6 gene transfection of mouse embryonic stem cells. Cornea, 26(10), 1220–1227.
da Mata Martins, T. M., da Silva Cunha, P., Rodrigues, M. A., de Carvalho, J. L., de Souza, J. E., de Carvalho Oliveira, J. A., et al. (2020). Epithelial basement membrane of human decellularized cornea as a suitable substrate for differentiation of embryonic stem cells into corneal epithelial-like cells. Materials Science and Engineering: C, 116, 111215.
Cieślar-Pobuda, A., Rafat, M., Knoflach, V., Skonieczna, M., Hudecki, A., Małecki, A., et al. (2016). Human induced pluripotent stem cell differentiation and direct transdifferentiation into corneal epithelial-like cells. Oncotarget, 7(27), 42314.
Mikhailova, A., Ilmarinen, T., Uusitalo, H., & Skottman, H. (2014). Small-molecule induction promotes corneal epithelial cell differentiation from human induced pluripotent stem cells. Stem Cell Reports, 2(2), 219–231.
Hongisto, H., Ilmarinen, T., Vattulainen, M., Mikhailova, A., & Skottman, H. (2017). Xeno-and feeder-free differentiation of human pluripotent stem cells to two distinct ocular epithelial cell types using simple modifications of one method. Stem Cell Research & Therapy, 8(1), 1–15.
Hongisto, H., Vattulainen, M., Ilmarinen, T., Mikhailova, A., & Skottman, H. (2018). Efficient and scalable directed differentiation of clinically compatible corneal limbal epithelial stem cells from human pluripotent stem cells. JoVE (Journal of Visualized Experiments), 140, e58279.
Kamarudin, T. A., Bojic, S., Collin, J., Yu, M., Alharthi, S., Buck, H., et al. (2018). Differences in the activity of endogenous bone morphogenetic protein signaling impact on the ability of induced pluripotent stem cells to differentiate to corneal epithelial-like cells. Stem Cells, 36(3), 337–348.
Hayashi, R., Ishikawa, Y., Katayama, T., Quantock, A. J., & Nishida, K. (2018). CD200 facilitates the isolation of corneal epithelial cells derived from human pluripotent stem cells. Scientific Reports, 8(1), 1–11.
Shibata, S., Hayashi, R., Kudo, Y., Okubo, T., Imaizumi, T., Katayama, T., et al. (2020). Cell-type-specific adhesiveness and proliferation propensity on laminin isoforms enable purification of iPSC-derived corneal epithelium. Stem Cell Reports, 14(4), 663–676.
Hayashi, R., Ishikawa, Y., Sasamoto, Y., Katori, R., Nomura, N., Ichikawa, T., et al. (2016). Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature, 531(7594), 376–380.
Hayashi, R., Ishikawa, Y., Katori, R., Sasamoto, Y., Taniwaki, Y., Takayanagi, H., et al. (2017). Coordinated generation of multiple ocular-like cell lineages and fabrication of functional corneal epithelial cell sheets from human iPS cells. Nature Protocols, 12(4), 683–696.
Shibata, S., Hayashi, R., Okubo, T., Kudo, Y., Katayama, T., Ishikawa, Y., et al. (2018). Selective laminin-directed differentiation of human induced pluripotent stem cells into distinct ocular lineages. Cell Reports, 25(6), 1668–1679.
Sareen, D., Saghizadeh, M., Ornelas, L., Winkler, M. A., Narwani, K., Sahabian, A., et al. (2014). Differentiation of human limbal-derived induced pluripotent stem cells into limbal-like epithelium. Stem Cells Translational Medicine, 3(9), 1002–1012.
Zhang, K., Pang, K., & Wu, X. (2014). Isolation and transplantation of corneal endothelial cell–like cells derived from in-vitro-differentiated human embryonic stem cells. Stem Cells and Development, 23(12), 1340–1354.
Chen, P., Chen, J. Z., Shao, C. Y., Li, C. Y., Zhang, Y. D., Lu, W. J., et al. (2015). Treatment with retinoic acid and lens epithelial cell-conditioned medium in vitro directed the differentiation of pluripotent stem cells towards corneal endothelial cell-like cells. Experimental and Therapeutic Medicine, 9(2), 351–360.
McCabe, K. L., Kunzevitzky, N. J., Chiswell, B. P., Xia, X., Goldberg, J. L., & Lanza, R. (2015). Efficient generation of human embryonic stem cell-derived corneal endothelial cells by directed differentiation. PLoS One, 10(12), e0145266.
Wagoner, M. D., Bohrer, L. R., Aldrich, B. T., Greiner, M. A., Mullins, R. F., Worthington, K. S., et al. (2018). Feeder-free differentiation of cells exhibiting characteristics of corneal endothelium from human induced pluripotent stem cells. Biology Open, 7(5), bio032102.
Grönroos, P., Ilmarinen, T., & Skottman, H. (2021). Directed differentiation of human pluripotent stem cells towards corneal endothelial-like cells under defined conditions. Cells, 10(2), 331.
Chan, A. A., Hertsenberg, A. J., Funderburgh, M. L., Mann, M. M., Du, Y., Davoli, K. A., et al. (2013). Differentiation of human embryonic stem cells into cells with corneal keratocyte phenotype. PloS one, 8(2), e56831.
Naylor, R. W., McGhee, C. N., Cowan, C. A., Davidson, A. J., Holm, T. M., & Sherwin, T. (2016). Derivation of corneal keratocyte-like cells from human induced pluripotent stem cells. PLoS ONE, 11(10), e0165464.
Joseph, R., Srivastava, O. P., & Pfister, R. R. (2016). Modeling keratoconus using induced pluripotent stem cells. Investigative Ophthalmology & Visual Science, 57(8), 3685–3697.
Aberdam, E., Petit, I., Sangari, L., & Aberdam, D. (2017). Induced pluripotent stem cell-derived limbal epithelial cells (LiPSC) as a cellular alternative for in vitro ocular toxicity testing. PLoS ONE, 12(6), e0179913.
Qin, S., Zheng, S., Qi, B., Guo, R., & Hou, G. (2019). Decellularized human stromal Lenticules combine with corneal epithelial-like cells: A new resource for corneal tissue engineering. Stem Cells International, 2019, 4252514.
Zhao, J. J., & Afshari, N. A. (2016). Generation of human corneal endothelial cells via in vitro ocular lineage restriction of pluripotent stem cells. Investigative Ophthalmology & Visual Science, 57(15), 6878–6884.
Ali, M., Khan, S. Y., Vasanth, S., Ahmed, M. R., Chen, R., Na, C. H., et al. (2018). Generation and proteome profiling of PBMC-originated, iPSC-derived corneal endothelial cells. Investigative Ophthalmology & Visual Science, 59(6), 2437–2444.
Itakura, G., Kawabata, S., Ando, M., Nishiyama, Y., Sugai, K., Ozaki, M., et al. (2017). Fail-safe system against potential tumorigenicity after transplantation of iPSC derivatives. Stem Cell Reports, 8(3), 673–684.
Singh, V. K., Kalsan, M., Kumar, N., Saini, A., & Chandra, R. (2015). Induced pluripotent stem cells: Applications in regenerative medicine, disease modeling, and drug discovery. Frontiers in Cell and Developmental Biology, 3, 2.
Pulimamidi, V. K., Maddileti, S., & Mariappan, I. (2021). Induced pluripotent stem-cell-derived corneal grafts and organoids. In iPSCs in Tissue Engineering (pp. 99–127). Academic Press. https://doi.org/10.1016/B978-0-12-823809-7.00005-0
Kitazawa, K., Hikichi, T., Nakamura, T., Mitsunaga, K., Tanaka, A., Nakamura, M., et al. (2016). OVOL2 maintains the transcriptional program of human corneal epithelium by suppressing epithelial-to-mesenchymal transition. Cell Reports, 15(6), 1359–1368.
Brejchova, K., Dudakova, L., Skalicka, P., Dobrovolny, R., Masek, P., Putzova, M., et al. (2019). IPSC-derived corneal endothelial-like cells act as an appropriate model system to assess the impact of SLC4A11 variants on pre-mRNA splicing. Investigative Ophthalmology & Visual Science, 60(8), 3084–3090.
Watanabe, S., Hayashi, R., Sasamoto, Y., Tsujikawa, M., Ksander, B. R., Frank, M. H., et al. (2021). Human iPS cells engender corneal epithelial stem cells with holoclone-forming capabilities. Iscience, 24(6), 102688.
Vattulainen, M., Ilmarinen, T., Viheriälä, T., Jokinen, V., & Skottman, H. (2021). Corneal epithelial differentiation of human pluripotent stem cells generates ABCB5+ and ∆Np63α+ cells with limbal cell characteristics and high wound healing capacity. Stem Cell Research & Therapy, 12(1), 1–17.
Vattulainen, M., Ilmarinen, T., Koivusalo, L., Viiri, K., Hongisto, H., & Skottman, H. (2019). Modulation of Wnt/BMP pathways during corneal differentiation of hPSC maintains ABCG2-positive LSC population that demonstrates increased regenerative potential. Stem Cell Research & Therapy, 10(1), 1–15.
West, J. D., Dorà, N. J., & Collinson, J. M. (2015). Evaluating alternative stem cell hypotheses for adult corneal epithelial maintenance. World Journal of Stem Cells, 7(2), 281.
Schlötzer-Schrehardt, U., & Kruse, F. E. (2005). Identification and characterization of limbal stem cells. Experimental Eye Research, 81(3), 247–264.
Guo, Z. H., Zhang, W., Jia, Y. Y. S., Liu, Q. X., Li, Z. F., & Lin, J. S. (2018). An insight into the difficulties in the discovery of specific biomarkers of limbal stem cells. International Journal of Molecular Sciences, 19(7), 1982.
Rama, P., Matuska, S., Paganoni, G., Spinelli, A., De Luca, M., & Pellegrini, G. (2010). Limbal stem-cell therapy and long-term corneal regeneration. New England Journal of Medicine, 363(2), 147–155.
de Paiva, C. S., Chen, Z., Corrales, R. M., Pflugfelder, S. C., & Li, D. Q. (2005). ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem cells, 23(1), 63–73.
Ksander, B. R., Kolovou, P. E., Wilson, B. J., Saab, K. R., Guo, Q., Ma, J., ... & Frank, N. Y. (2014). ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature, 511(7509), 353-357.
Schlereth, S. L., Hos, D., Matthaei, M., Hamrah, P., Schmetterer, L., O’Leary, O., ... & Cursiefen, C. (2021). New technologies in clinical trials in corneal diseases and limbal stem cell deficiency: review from the European Vision Institute Special Interest Focus Group meeting. Ophthalmic Research, 64(2), 145-167.
Singh, V., Tiwari, A., Kethiri, A. R., & Sangwan, V. S. (2021). Current perspectives of limbal-derived stem cells and its application in ocular surface regeneration and limbal stem cell transplantation. Stem Cells Translational Medicine, 10(8), 1121–1128.
Kate, A., & Basu, S. (2022). A Review of the Diagnosis and Treatment of Limbal Stem Cell Deficiency. Frontiers in Medicine, 9, 836009.
Sangwan, V. S., Basu, S., MacNeil, S., & Balasubramanian, D. (2012). Simple limbal epithelial transplantation (SLET): A novel surgical technique for the treatment of unilateral limbal stem cell deficiency. British Journal of Ophthalmology, 96(7), 931–934.
Thokala, P., Singh, A., Singh, V. K., Rathi, V. M., Basu, S., Singh, V., ... & Sangwan, V. S. (2021). Economic, clinical and social impact of simple limbal epithelial transplantation for limbal stem cell deficiency.British Journal of Ophthalmology, 106, 923-928.
Shanbhag, S. S., Patel, C. N., Goyal, R., Donthineni, P. R., Singh, V., & Basu, S. (2019). Simple limbal epithelial transplantation (SLET): Review of indications, surgical technique, mechanism, outcomes, limitations, and impact. Indian Journal of Ophthalmology, 67(8), 1265–1277.
Ghareeb, A. E., Lako, M., & Figueiredo, F. C. (2020). Recent advances in stem cell therapy for limbal stem cell deficiency: A narrative review. Ophthalmology and Therapy, 9(4), 809–831.
Vazirani, J., Mariappan, I., Ramamurthy, S., Fatima, S., Basu, S., & Sangwan, V. S. (2016). Surgical management of bilateral limbal stem cell deficiency. The Ocular Surface, 14(3), 350–364.
Sun, B., Bikkuzin, T., Li, X., Shi, Y., & Zhang, H. (2021). Human-Induced Pluripotent Stem Cells-Derived Corneal Endothelial-Like Cells Promote Corneal Transparency in a Rabbit Model of Bullous Keratopathy. Stem Cells and Development, 30(17), 856–864.
Ali, M., Khan, S. Y., Gottsch, J. D., Hutchinson, E. K., Khan, A., & Riazuddin, S. A. (2021). Pluripotent stem cell–derived corneal endothelial cells as an alternative to donor corneal endothelium in keratoplasty. Stem Cell Reports, 16(9), 2320–2335.
Hatou, S., Sayano, T., Higa, K., Inagaki, E., Okano, Y., Sato, Y., et al. (2021). Transplantation of iPSC-derived corneal endothelial substitutes in a monkey corneal edema model. Stem Cell Research, 55, 102497.
Cyranoski, D. (2019). Japan poised to allow’reprogrammed’stem-cell therapy for damaged corneas. Nature. https://doi.org/10.1038/d41586-019-00860-0
Japan team proves iPS-based cornea transplant safe in world-1st trial. (2022). Kyodo News. https://english.kyodonews.net/news/2022/04/c8af6b7913b2-japan-team-proves-ips-based-cornea-transplant-safe-in-world-1st-trial.html. Accessed 20 May 2022
Sorkio, A., Koch, L., Koivusalo, L., Deiwick, A., Miettinen, S., Chichkov, B., & Skottman, H. (2018). Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials, 171, 57–71.
Anton-Sales, I., Koivusalo, L., Skottman, H., Laromaine, A., & Roig, A. (2021). Limbal stem cells on bacterial nanocellulose carriers for ocular surface regeneration. Small (Weinheim an der Bergstrasse, Germany), 17(10), 2003937.
Koivusalo, L., Kauppila, M., Samanta, S., Parihar, V. S., Ilmarinen, T., Miettinen, S., et al. (2019). Tissue adhesive hyaluronic acid hydrogels for sutureless stem cell delivery and regeneration of corneal epithelium and stroma. Biomaterials, 225, 119516.
Ahearne, M., Fernández-Pérez, J., Masterton, S., Madden, P. W., & Bhattacharjee, P. (2020). Designing scaffolds for corneal regeneration. Advanced Functional Materials, 30(44), 1908996.
Mikhailova, A., Ilmarinen, T., Ratnayake, A., Petrovski, G., Uusitalo, H., Skottman, H., & Rafat, M. (2016). Human pluripotent stem cell-derived limbal epithelial stem cells on bioengineered matrices for corneal reconstruction. Experimental Eye Research, 146, 26–34.
Chakrabarty, K., Shetty, R., & Ghosh, A. (2018). Corneal cell therapy: With iPSCs, it is no more a far-sight. Stem Cell Research & Therapy, 9(1), 1–15.
Choi, H. W., Kim, J. S., Choi, S., Hong, Y. J., Kim, M. J., Seo, H. G., & Do, J. T. (2014). Neural stem cells differentiated from iPS cells spontaneously regain pluripotency. Stem Cells, 32(10), 2596–2604.
Lu, X., & Zhao, T. (2013). Clinical therapy using iPSCs: Hopes and challenges. Genomics, proteomics & bioinformatics, 11(5), 294–298.
Malik, N., & Rao, M. S. (2013). A review of the methods for human iPSC derivation. Pluripotent Stem Cells, 23–33. https://doi.org/10.1007/978-1-62703-348-0_3
Ji, P., Manupipatpong, S., Xie, N., & Li, Y. (2016). Induced pluripotent stem cells: generation strategy and epigenetic mystery behind reprogramming. Stem Cells International, 2016. https://doi.org/10.1155/2016/8415010
González, F., Boué, S., & Belmonte, J. C. I. (2011). Methods for making induced pluripotent stem cells: Reprogramming a la carte. Nature Reviews Genetics, 12(4), 231–242.
Mora, C., Serzanti, M., Consiglio, A., Memo, M., & Dell’Era, P. (2017). Clinical potentials of human pluripotent stem cells. Cell Biology and Toxicology, 33(4), 351–360.
Kim, K., Wen, B., Ng, K., Zhao, R., Cahan, P., Kim, J., et al. (2010). Epigenetic memory in induced pluripotent stem cells. Nature, 467(7313), 285–290.
Nishizawa, M., Chonabayashi, K., Nomura, M., Tanaka, A., Nakamura, M., Inagaki, A., et al. (2016). Epigenetic variation between human induced pluripotent stem cell lines is an indicator of differentiation capacity. Cell Stem Cell, 19(3), 341–354.
Vitale, A. M., Matigian, N. A., Ravishankar, S., Bellette, B., Wood, S. A., Wolvetang, E. J., & Mackay-Sim, A. (2012). Variability in the generation of induced pluripotent stem cells: Importance for disease modeling. Stem Cells Translational Medicine, 1(9), 641–650.
Grobarczyk, B., Franco, B., Hanon, K., & Malgrange, B. (2015). Generation of isogenic human iPS cell line precisely corrected by genome editing using the CRISPR/Cas9 system. Stem Cell Reviews and Reports, 11(5), 774–787.
Zhang, G., Shang, B., Yang, P., Cao, Z., Pan, Y., & Zhou, Q. (2012). Induced pluripotent stem cell consensus genes: Implication for the risk of tumorigenesis and cancers in induced pluripotent stem cell therapy. Stem Cells and Development, 21(6), 955–964.
Gutierrez-Aranda, I., Ramos-Mejia, V., Bueno, C., Munoz-Lopez, M., Real, P. J., Mácia, A., et al. (2010). Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells, 28(9), 1568–1570.
Ghosh, Z., Huang, M., Hu, S., Wilson, K. D., Dey, D., & Wu, J. C. (2011). Dissecting the oncogenic and tumorigenic potential of differentiated human induced pluripotent stem cells and human embryonic stem cells. Cancer Research, 71(14), 5030–5039.
Mitsui, K., Ide, K., Takahashi, T., & Kosai, K. I. (2017). Viral vector-based innovative approaches to directly abolishing tumorigenic pluripotent stem cells for safer regenerative medicine. Molecular Therapy-Methods & Clinical Development, 5, 51–58.
Ben-David, U., Nudel, N., & Benvenisty, N. (2013). Immunologic and chemical targeting of the tight-junction protein Claudin-6 eliminates tumorigenic human pluripotent stem cells. Nature Communications, 4(1), 1–8.
Neofytou, E., O’Brien, C. G., Couture, L. A., & Wu, J. C. (2015). Hurdles to clinical translation of human induced pluripotent stem cells. The Journal of Cinical Investigation, 125(7), 2551–2557.
Bravery, C. A. (2015). Do human leukocyte antigen-typed cellular therapeutics based on induced pluripotent stem cells make commercial sense? Stem Cells and Development, 24(1), 1–10.
Devito, L., Petrova, A., Miere, C., Codognotto, S., Blakely, N., Lovatt, A., et al. (2014). Cost-effective master cell bank validation of multiple clinical-grade human pluripotent stem cell lines from a single donor. Stem Cells Translational Medicine, 3(10), 1116–1124.
Koga, K., Wang, B., & Kaneko, S. (2020). Current status and future perspectives of HLA-edited induced pluripotent stem cells. Inflammation and Regeneration, 40(1), 1–6.
Nakatsuji, N., Nakajima, F., & Tokunaga, K. (2008). HLA-haplotype banking and iPS cells. Nature Biotechnology, 26(7), 739–740.
Taylor, C. J., Peacock, S., Chaudhry, A. N., Bradley, J. A., & Bolton, E. M. (2012). Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell, 11(2), 147–152.
Volpato, V., & Webber, C. (2020). Addressing variability in iPSC-derived models of human disease: Guidelines to promote reproducibility. Disease Models & Mechanisms, 13(1), dmm042317.
Thompson, S. D. (2014). Scientific innovation’s two valleys of death: How blood and tissue banks can help to bridge the gap. Stem Cells and Development, 23(S1), 68–72.
Butler, D. (2008). Translational research: Crossing the valley of death. Nature, 453, 840–842.
Pashuck, E. T., & Stevens, M. M. (2012). Designing regenerative biomaterial therapies for the clinic. Science Translational Medicine, 4(160sr4).
Trounson, A., Baum, E., Gibbons, D., & Tekamp-Olson, P. (2010). Developing a case study model for successful translation of stem cell therapies. Cell Stem Cell, 6(6), 513–516.
Trounson, A., DeWitt, N. D., & Feigal, E. G. (2012). The Alpha Stem Cell Clinic: A model for evaluating and delivering stem cell-based therapies. Stem Cells Translational Medicine, 1(1), 9–14.
Funding
This work was supported by the Wilson College of Textiles (JMG), the Department of Textile Engineering, Chemistry and Science (JMG), North Carolina Textile Foundation-Wilson College Fellowship (NM), NCSU Provost’s Fellowship (TCS). Graphics were created with BioRender.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors have no commercial associations or conflicts of interest to disclose.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mahmood, N., Suh, T.C., Ali, K.M. et al. Induced Pluripotent Stem Cell-Derived Corneal Cells: Current Status and Application. Stem Cell Rev and Rep 18, 2817–2832 (2022). https://doi.org/10.1007/s12015-022-10435-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10435-8