Abstract
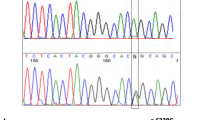
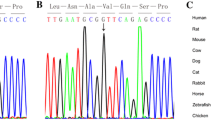
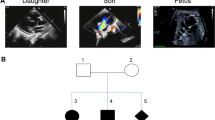
Mutations of NKX2-5 largely contribute to congenital heart diseases (CHDs), especially atrial septal defect (ASD). We identified a novel heterozygous splicing mutation c.335-1G > A in NKX2-5 gene in an ASD family via whole exome sequencing (WES) and linkage analysis. Utilizing the human induced pluripotent stem cell (hiPSC)-derived cardiomyocytes (hiPSC-CMs) as a disease model, we showed that haploinsufficiency of NKX2-5 contributed to aberrant orchestration of apoptosis and proliferation in ASD patient-derived hiPSC-CMs. RNA-seq profiling and dual-luciferase reporter assay revealed that NKX2-5 acts upstream of PYK2 via miR-19a and miR-19b (miR-19a/b) to regulate cardiomyocyte apoptosis. Meanwhile, miR-19a/b are also downstream mediators of NKX2-5 during cardiomyocyte proliferation. The novel splicing mutation c.335-1G > A in NKX2-5 and its potential pathogenic roles in ASD were demonstrated. Our work provides clues not only for deep understanding of NKX2-5 in cardia development, but also for better knowledge in the molecular mechanisms of CHDs.
Graphical abstract







Similar content being viewed by others
Availability of Data and Material
The datasets in this study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Bouma, B. J., & Mulder, B. J. M. (2017). Changing landscape of congenital heart disease. Circulation Research, 120(6), 908–922.
van der Linde, D., Konings, E. E. M., Slager, M. A., Witsenburg, M., Helbing, W. A., Takkenberg, J. J. M., & Roos-Hesselink, J. W. (2011). Birth prevalence of congenital heart disease worldwide. Journal of the American College of Cardiology, 58(21), 2241–2247.
Tal, G., Jose, D. M., & Wald, R. M. (2014). Atrial septal defects. Lancet, 383, 1921–1932.
Ye, L., Yu, Y., Zhao, Z., Zhao, D., Ni, X., Wang, Y., Fang, X., Yu, M., Wang, Y., Tang, J., Chen, Y., Shen, Z., Lei, W., & Hu, S. (2022). Patient-specific iPSC-derived cardiomyocytes reveal abnormal regulation of FGF16 in a familial atrial septal defect. Cardiovascular Research, 118(3), 859–871.
Huang, S., Wu, Y., Chen, S., Yang, Y., Wang, Y., Wang, H., Li, P., Zhuang, J., & Xia, Y. (2021). Novel insertion mutation (Arg1822_Glu1823dup) in MYH6 coiled-coil domain causing familial atrial septal defect. European Journal of Medical Genetics, 64(11), 104314.
Frank, D., Rangrez, A. Y., Friedrich, C., Dittmann, S., Stallmeyer, B., Yadav, P., Bernt, A., Schulze-Bahr, E., Borlepawar, A., Zimmermann, W., Peischard, S., Seebohm, G., Linke, W. A., Baba, H. A., Krüger, M., Unger, A., Usinger, P., Frey, N., & Schulze-Bahr, E. (2019). Cardiac α-Actin (ACTC1) gene mutation causes Atrial-Septal defects associated with Late-Onset dilated cardiomyopathy. Circulation: Genomic and Precision Medicine, 12(8), e2491.
James, T. N. (1997). Apoptosis in congenital heart disease. Coronary Artery Disease, 8(10), 599–616.
Pierpont, M. E., Brueckner, M., Chung, W. K., Garg, V., Lacro, R. V., McGuire, A. L., Mital, S., Priest, J. R., Pu, W. T., Roberts, A., Ware, S. M., Gelb, B. D., & Russell, M. W. (2018). Genetic basis for congenital heart disease: Revisited: A scientific statement from the american heart association. Circulation, 138(21), e653–e711.
Schott, J., Benson, D. W., Basson, C. T., Pease, W., Silberbach, G. M., Moak, J. P., Maron, B. J., Seidman, C. E., & Seidmans, J. G. (1998). Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science, 5373(281), 108–111.
Garg, V., Kathiriya, I. S., Barnes, R., Schluterman, M. K., King, I. N., Butler, C. A., Rothrock, C. R., Eapen, R. S., Hirayama-Yamada, K., Joo, K., Matsuoka, R., Cohen, J. C., & Srivastava, D. (2003). GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature, 424(6947), 443–447.
Akazawa, H., & Komuro, I. (2005). Cardiac transcription factor Csx/Nkx2-5: Its role in cardiac development and diseases. Pharmacology & Therapeutics, 107(2), 252–268.
Kasahara, H., & Benson, D. W. (2004). Biochemical analyses of eight NKX2.5 homeodomain missense mutations causing atrioventricular block and cardiac anomalies. Cardiovascular Research, 64(1), 40–51.
Su, W., Zhu, P., Wang, R., Wu, Q., Wang, M., Zhang, X., Mei, L., Tang, J., Kumar, M., Wang, X., Su, L., & Dong, N. (2016). Congenital heart diseases and their association with the variant distribution features on susceptibility genes. Clinical Genetics, 91(3), 349–354.
Ashraf, H., Pradhan, L., Chang, E. I., Terada, R., Ryan, N. J., Briggs, L. E., Chowdhury, R., Zárate, M. A., Sugi, Y., Nam, H., Benson, D. W., Anderson, R. H., & Kasahara, H. (2014). A mouse model of human congenital heart disease. Circulation: Cardiovascular Genetics, 7(4), 423–433.
Anderson, D. J., Kaplan, D. I., Bell, K. M., Koutsis, K., Haynes, J. M., Mills, R. J., Phelan, D. G., Qian, E. L., Leitoguinho, A. R., Arasaratnam, D., Labonne, T., Ng, E. S., Davis, R. P., Casini, S., Passier, R., Hudson, J. E., Porrello, E. R., Costa, M. W., Rafii, A., Curl, C. L., Delbridge, L. M., Harvey, R. P., Oshlack, A., Cheung, M. M., Mummery, C. L., Petrou, S., Elefanty, A. G., Stanley, E. G., & Elliott, D. A. (2018). NKX2–5 regulates human cardiomyogenesis via a HEY2 dependent transcriptional network. Nature Communications, 9(1).
Sarkozy, A., Conti, E., Neri, C., Agostino, R. D., Digilio, M. C., Esposito, G., Toscano, A., Marino, B., Pizzuti, A., & Dallapiccola, B. (2005). Spectrum of atrial septal defects associated with mutations of NKX2.5 and GATA4 transcription factors. Journal of Medical Genetics, 42(2), e16.
Abou Hassan, O. K., Fahed, A. C., Batrawi, M., Arabi, M., Refaat, M. M., DePalma, S. R., Seidman, J. G., Seidman, C. E., Bitar, F. F., & Nemer, G. M. (2015). NKX2-5 mutations in an inbred consanguineous population: Genetic and phenotypic diversity. Scientific Reports, 5, 8848.
Biben, C., Weber, R., Kesteven, S., Stanley, E., McDonald, L., Elliott, D. A., Barnett, L., Koentgen, F., Robb, L., Feneley, M., & Harvey, R. P. (2000). Cardiac septal and valvular dysmorphogenesis in mice heterozygous for mutations in the homeobox gene Nkx2-5. Circulation Research, 87(10), 888–895.
Zhang, D., Dai, L., Zhou, Z., Hu, J., Bai, Y., & Guo, H. (2019). Homozygosity mapping and whole exome sequencing reveal a novel ERCC8 mutation in a Chinese consanguineous family with unique cerebellar ataxia. Clinica Chimica Acta, 494, 64–70.
Dai, L., Zhang, D., Wu, Z., Guan, X., Ma, M., Li, L., Zhang, Y., Bai, Y., & Guo, H. (2021). A tiered genetic screening strategy for the molecular diagnosis of intellectual disability in chinese patients. Frontiers in Genetics, 12.
Burridge, P. W., Holmström, A., & Wu, J. C. (2015). Chemically defined culture and cardiomyocyte differentiation of human pluripotent stem cells. Current Protocols in Human Genetics, 87(1).
Basile, J. R., Afkhami, T., & Gutkind, J. S. (2005). Semaphorin 4D/Plexin-B1 induces endothelial cell migration through the activation of PYK2, src, and the phosphatidylinositol 3-Kinase-Akt pathway. Molecular and Cellular Biology, 25(16), 6889–6898.
Miyazaki, T., Takaoka, A., Nogueira, L., Dikic, I., Fujii, H., Tsujino, S., Mitani, Y., Maeda, M., Schlessinger, J., & Taniguchi, T. (1998). Pyk2 is a downstream mediator of the IL-2 receptor-coupled Jak signaling pathway. Genes & Development, 12(6), 770–775.
Ohtsu, H., Mifune, M., Frank, G. D., Saito, S., Inagami, T., Kim-Mitsuyama, S., Takuwa, Y., Sasaki, T., Rothstein, J. D., Suzuki, H., Nakashima, H., Woolfolk, E. A., Motley, E. D., & Eguchi, S. (2005). Signal-crosstalk between Rho/ROCK and c-Jun NH2-terminal kinase mediates migration of vascular smooth muscle cells stimulated by angiotensin II. Arteriosclerosis, Thrombosis, and Vascular Biology, 25(9), 1831–1836.
Burdick, A. D., Ivnitski-Steele, I. D., Lauer, F. T., & Burchiel, S. W. (2006). PYK2 mediates anti-apoptotic AKT signaling in response to benzo[a]pyrene diol epoxide in mammary epithelial cells. Carcinogenesis (New York), 27(11), 2331–2340.
Dikic, I., Ivankovic-Dikic, I., Grönroos, E., Blaukat, A., & Barth, B. (2000). Pyk2 and FAK regulate neurite outgrowth induced by growth factors and integrins. Nature Cell Biology, 2(9), 574–581.
Rajewsky, N., Krek, A., Grün, D., Poy, M. N., Wolf, R., Rosenberg, L., Epstein, E. J., MacMenamin, P., Da Piedade, I., Gunsalus, K. C., & Stoffel, M. (2005). Combinatorial microRNA target predictions. Nature Genetics, 37(5), 495–500.
Agarwal, V., Bell, G., Nam, J., & Bartel, D. (2015). Predicting efective microRNA target sites in mammalian mRNAs. eLife, 4, e5005.
McGeary, S. E., Lin, K. S., Shi, C. Y., Pham, T. M., Bisaria, N., Kelley, G. M., & Bartel, D. P. (2019). The biochemical basis of microRNA targeting efficacy. Science, 366(6472).
Li, J., Liu, S., Zhou, H., Qu, L., & Yang, J. (2014). StarBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Research, 42, D92–D97.
Bardou, P., Mariette, J., Escudié, F., Djemiel, C., & Klopp, C. (2014). Jvenn: An interactive Venn diagram viewer. BMC Bioinformatics, 15(1), 293.
Ventura, A., Young, A. G., Winslow, M. M., Lintault, L., Meissner, A., Erkeland, S. J., Newman, J., Bronson, R. T., Crowley, D., Stone, J. R., Jaenisch, R., Sharp, P. A., & Jacks, T. (2008). Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell, 132(5), 875–886.
Stefan, N., Letizia, V., Przybylski, G. K., Piotr, G., Schmidt, C. A., Corinna, M., Drexler, H. G., Macleod, R. A. F., & Scherr, M. (2009). Activation of miR-17-92 by NK-like homeodomain proteins suppresses apoptosis via reduction of E2F1 in T-cell acute lymphoblastic leukemia. Leukemia & Lymphoma, 1(50), 101–108.
Gao, F., Kataoka, M., Liu, N., Liang, T., Huang, Z., Gu, F., Ding, J., Liu, J., Zhang, F., Ma, Q., Wang, Y., Zhang, M., Hu, X., Kyselovic, J., Hu, X., Pu, W. T., Wang, J. A., Chen, J., & Wang, D. (2019). Therapeutic role of miR-19a/19b in cardiac regeneration and protection from myocardial infarction. Nature Communications, 10(1).
Zhang, G. F., Zhong, J. M., Lin, L., & Liu, Z. H. (2020). MiR-19 enhances pancreatic cancer progression by targeting PTEN through PI3K/AKT signaling pathway. European Review for Medical and Pharmacological Sciences, 24(3), 1098–1107.
Li, X., Xie, W., Xie, C., Huang, C., Zhu, J., Liang, Z., Deng, F., Zhu, M., Zhu, W., Wu, R., Wu, J., Geng, S., & Zhong, C. (2014). Curcumin modulates miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7 breast cancer cell proliferation. Phytotherapy Research, 28(10), 1553–1560.
MacGrogan, D., Münch, J., & de la Pompa, J. L. (2018). Notch and interacting signalling pathways in cardiac development, disease, and regeneration. Nature Reviews Cardiology, 15(11), 685–704.
Banerjee, I., Carrion, K., Serrano, R., Dyo, J., Sasik, R., Lund, S., Willems, E., Aceves, S., Meili, R., Mercola, M., Chen, J., Zambon, A., Hardiman, G., Doherty, T. A., Lange, S., Del Álamo, J. C., & Nigam, V. (2015). Cyclic stretch of embryonic cardiomyocytes increases proliferation, growth, and expression while repressing Tgf-β signaling. Journal of Molecular and Cellular Cardiology, 79, 133–144.
Shyu, K. (2009). Cellular and molecular effects of mechanical stretch on vascular cells and cardiac myocytes. Clinical Science, 116(5), 377–389.
Torsoni, A. S., Marin, T. M., Velloso, L. A., & Franchini, K. G. (2005). RhoA/ROCK signaling is critical to FAK activation by cyclic stretch in cardiac myocytes. American Journal of Physiology-Heart and Circulatory Physiology, 289(4), H1488–H1496.
Schaller, M. D. (2010). Cellular functions of FAK kinases: Insight into molecular mechanisms and novel functions. Journal of Cell Science, 123(Pt 7), 1007–1013.
Davies, P. F. (2002). Editorial multiple signaling pathways in Flow-Mediated endothelial mechanotransduction. Arteriosclerosis, Thrombosis, and Vascular Biology, 22(11), 1755–1757.
Heidkamp, M. C., Scully, B. T., Vijayan, K., Engman, S. J., Szotek, E. L., & Samarel, A. M. (2005). PYK2 regulates SERCA2 gene expression in neonatal rat ventricular myocytes. American Journal of Physiology-Cell Physiology, 289(2), C471–C482.
Murphy, J. M., Jeong, K., Rodriguez, Y., Kim, J. H., Ahn, E. E., & Lim, S. S. (2019). FAK and Pyk2 activity promote TNF-alpha and IL-1beta-mediated pro-inflammatory gene expression and vascular inflammation. Science and Reports, 9(1), 7617.
Melendez, J., Turner, C., Avraham, H., Steinberg, S. F., Schaefer, E., & Sussman, M. A. (2004). Cardiomyocyte apoptosis triggered by RAFTK/pyk2 via src kinase is antagonized by paxillin. Journal of Biological Chemistry, 279(51), 53516–53523.
Canobbio, I., Cipolla, L., Consonni, A., Momi, S., Guidetti, G., Oliviero, B., Falasca, M., Okigaki, M., Balduini, C., Gresele, P., & Torti, M. (2013). Impaired thrombin-induced platelet activation and thrombus formation in mice lacking the Ca2+-dependent tyrosine kinase Pyk2. Blood, 121(4), 648–657.
Lang, D., Glukhov, A. V., Efimova, T., & Efimov, I. R. (2011). Role of Pyk2 in cardiac arrhythmogenesis. American Journal of Physiology-Heart and Circulatory Physiology, 301(3), H975–H983.
Toko, H., Zhu, W., Takimoto, E., Shiojima, I., Hiroi, Y., Zou, Y., Oka, T., Akazawa, H., Mizukami, M., Sakamoto, M., Terasaki, F., Kitaura, Y., Takano, H., Nagai, T., Nagai, R., & Komuro, I. (2002). Csx/Nkx2-5 is required for homeostasis and survival of cardiac myocytes in the adult heart. Journal of Biological Chemistry, 277(27), 24735–24743.
Prall, O. W., Menon, M. K., Solloway, M. J., Watanabe, Y., Zaffran, S., Bajolle, F., Biben, C., McBride, J. J., Robertson, B. R., Chaulet, H., Stennard, F. A., Wise, N., Schaft, D., Wolstein, O., Furtado, M. B., Shiratori, H., Chien, K. R., Hamada, H., Black, B. L., … Harvey, R. P. (2007). An Nkx2-5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell, 128(5), 947–959.
Nakashima, Y., Yanez, D. A., Touma, M., Nakano, H., Jaroszewicz, A., Jordan, M. C., Pellegrini, M., Roos, K. P., & Nakano, A. (2014). Nkx2-5 suppresses the proliferation of atrial myocytes and conduction system. Circulation Research, 114(7), 1103–1113.
Zheng, W., Lu, Y., Liang, S., Zhang, Q., Xu, J., She, Z., Zhang, Z., Yang, R., Mao, B., Xu, Z., Li, L., Hao, D., Lu, J., Wei, Y., Chen, H., & Liu, D. (2013). SIRT1 mediates the protective function of Nkx2.5 during stress in cardiomyocytes. Basic Research in Cardiology, 108(4).
Schott, J. J., Benson, D. W., Basson, C. T., Pease, W., Silberbach, G. M., Moak, J. P., Maron, B. J., Seidman, C. E., & Seidman, J. G. (1998). Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science, 281(5373), 108–111.
Lyons, I., Parsons, L. M., Hartley, L., Li, R., Andrews, J. E., Robb, L., & Harvey, R. P. (1995). Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes & Development, 9(13), 1566–1654.
Bouveret, R., Waardenberg, A. J., Schonrock, N., Ramialison, M., Doan, T., de Jong, D., Bondue, A., Kaur, G., Mohamed, S., Fonoudi, H., Chen, C., Wouters, M. A., Bhattacharya, S., Plachta, N., Dunwoodie, S. L., Chapman, G., Blanpain, C., & Harvey, R. P. (2015). NKX2–5 mutations causative for congenital heart disease retain functionality and are directed to hundreds of targets. ELife, 4.
Zhu, W., Shiojima, I., Hiroi, Y., Zou, Y., Akazawa, H., Mizukami, M., Toko, H., Yazaki, Y., Nagai, R., & Komuro, I. (2000). Functional analyses of three Csx/Nkx-2.5 mutations that cause human congenital heart disease. Journal of Biological Chemistry, 275(45), 35291–35296.
Kasahara, H., Lee, B., Schott, J. J., Benson, D. W., Seidman, J. G., Seidman, C. E., & Izumo, S. (2000). Loss of function and inhibitory effects of human CSX/NKX2.5 homeoprotein mutations associated with congenital heart disease. Journal of Clinical Investigation, 106(2), 299–308.
Watada, H., Mirmira, R. G., Kalamaras, J., & German, M. S. (2000). Intramolecular control of transcriptional activity by the NK2-specific domain in NK-2 homeodomain proteins. Proc Natl Acad Sci U S A, 97(17), 9443–9448.
Ouyang, P., Saarel, E., Bai, Y., Luo, C., Lv, Q., Xu, Y., Wang, F., Fan, C., Younoszai, A., Chen, Q., Tu, X., & Wang, Q. K. (2011). A de novo mutation in NKX2.5 associated with atrial septal defects, ventricular noncompaction, syncope and sudden death. Clinica Chimica Acta, 412(1–2), 170–175.
Olson, E. N. (2006). Gene regulatory networks in the evolution and development of the heart. Science, 313(5795), 1922–1927.
Mishra, P. K., Tyagi, N., Kumar, M., & Tyagi, S. C. (2009). MicroRNAs as a therapeutic target for cardiovascular diseases. Journal of Cellular and Molecular Medicine, 13(4), 778–789.
Ha, M., & Kim, V. N. (2014). Regulation of microRNA biogenesis. Nature Reviews Molecular Cell Biology, 15(8), 509–524.
Chen, J., Huang, Z., Seok, H. Y., Ding, J., Kataoka, M., Zhang, Z., Hu, X., Wang, G., Lin, Z., Wang, S., Pu, W. T., Liao, R., & Wang, D. (2013). Mir-17–92 Cluster is Required for and Sufficient to Induce Cardiomyocyte Proliferation in Postnatal and Adult Hearts. Circulation Research, 112(12), 1557–1566.
Yang, W., Han, Y., Yang, C., Chen, Y., Zhao, W., Su, X., Yang, K., & Jin, W. (2019). MicroRNA-19b-1 reverses ischaemia-induced heart failure by inhibiting cardiomyocyte apoptosis and targeting Bcl2 l11/BIM. Heart and Vessels, 34(7), 1221–1229.
Gao, Y., Qian, J., Chen, Z., Fu, M., Xu, J., Xia, Y., Ding, X., Yang, X., Cao, Y., Zou, Y., Ren, J., Ai-Jun, S., & Ge, J. (2016). Suppression of Bim by microRNA-19a may protect cardiomyocytes against hypoxia-induced cell death via autophagy activation. Toxicology Letters, 257, 72–83.
Acknowledgements
The authors would like to thank Cellapy Corporation (Beijing, China) for providing WT hiPSC. We also thank the family members for participating in this study. Some or all data, models, or code generated or used during the study are available from the corresponding author by request.
Funding
This work was supported by National Natural Science Foundation of China (No. 81100068 and 81570217), Natural Science Foundation Project of Chongqing (cstc2018jcyjAX0641 and cstc2021jcyj-msxmX0281).
Author information
Authors and Affiliations
Contributions
Guo H and Bai Y designed and supervised the study. Guo H reviewed and amended the manuscript. Li J prepared the manuscript, generated CMs from hiPSCs, evaluated the apoptosis and proliferation of the hiPSC-CMs, did the reporter assay and WB. Dai LM did the linkage analysis and analyzed the WES and RNASeq data. Tan XY generated the hiPSCs model. Wang JW and Zhu XT cultured the cells and differentiation. Xiong G collected the samples and the clinical data of the family.
Corresponding authors
Ethics declarations
Ethics Approval
This study was performed with the approval of the Ethics Committee of Army Medical University (Third Military Medical University), Chongqing, China and with the 1964 Helsinki Declaration.
Consent to Participate
Written statements of informed consent were obtained from the participants or legal guardians in the study.
Consent for Publication
All participants or their legal guardians in this study gave their consent for information to be published.
Conflicts of Interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12015_2022_10400_MOESM1_ESM.pptx
Supplementary figure 1 (A) Sanger sequencing chromatograms of 3 kinds of NKX2-5transcripts in hiPSC-CMs of family member V2. The dotted box indicated the 2bp deletion at the beginning of exon2 in transcript C. (B and C) Protein expression levels andquantification of NKX2-5 in WT and ASD hiPSC-CMs at day 20, 25 and 28 withGAPDH as a loading control. (**P≤0.01; *** P≤0.001;ns, no significance; Student’s t-test, n=3 independent experiments) (PPTX 3559 KB)
Rights and permissions
About this article
Cite this article
Jia, L., Limeng, D., Xiaoyin, T. et al. A Novel Splicing Mutation c.335–1 G > A in the Cardiac Transcription Factor NKX2-5 Leads to Familial Atrial Septal Defect Through miR-19 and PYK2. Stem Cell Rev and Rep 18, 2646–2661 (2022). https://doi.org/10.1007/s12015-022-10400-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10400-5