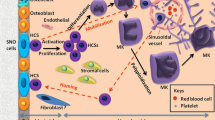
Abstract
Platelets (PLTs) are small anucleate blood cells that release from polyploidy megakaryocytes(MKs). PLT transfusion is standard therapy to prevent hemorrhage. PLT transfusion is donor‐dependent way which have limitations including the inadequate donor blood supply, poor quality, and issues related to infection and immunity. Overcoming these obstacles is possible with in vitro production of human PLTs. Currently several cells have been considered as source to in vitro production of PLTs such as hematopoietic stem cells (HSCs), embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). However, HSCs are a limited source for PLT production and large-scale expansion of HSC-derived PLT remains difficult. Alternative sources can be ESCs which have unlimited expansion capacity. But ESCs have ethical issues related to destroying human embryos. iPSCs are considered as an ideal unlimited source for PLT production. They are able to differentiate into any cells and have the capacity of self-renewal. Moreover, iPSCs can be acquired from any donor and easily manipulated. Due to new advances in development of MK cell lines, bioreactors, feeder cell-free production and the ability of large scale generation, iPSC-based PLTs are moving toward clinical applicability and considering the minimal risk of alloimmunization and tumorigenesis of these products, there is great hopefulness they will become the standard source for blood transfusions in the future. This review will focus on how to progress of in vitro generation of PLT from stem cell especially iPSCs and some of the successful strategies that can be easily used in clinic will be described.
Graphical Abstract




Similar content being viewed by others
Availability of Data and Materials
Not applicable.
Abbreviations
- iPSCs:
-
Induced Pluripotent Stem Cells
- hiPSC:
-
Human Induced Pluripotent Stem Cells
- PLTs:
-
Platelets
- MKs:
-
Megakaryocytes
- PRP:
-
Platelet-Rich Plasma
- BM:
-
Bone marrow
- PB:
-
Peripheral Blood
- HLA:
-
Human Leukocyte Antigen
- HPA:
-
Human Platelet Antigen
- hPSCs:
-
Human pluripotent stem cells
- PTR:
-
Platelets Transfusion Refractoriness
- TRALI:
-
Transfusion-Related Acute Lung Injury
- UCB:
-
Umbilical Cord Blood
- HSCs:
-
Hematopoietic stem cells
- TPO:
-
Thrombopoietin
- SCF:
-
Stem Cell Factor
- ESCs:
-
Embryonic Stem Cells
- hESCs:
-
Human Embryonic Stem Cells
- VEGF:
-
Vascular Endothelial Growth Factor
- ES-sacs:
-
Embryonic Stem Cell–Derived Sacs
- HPCs:
-
Hematopoietic Progenitor Cells
- TF:
-
Transcription Factors
- YFs:
-
Yamanaka factors
- EB:
-
Embryoid Body
- MEF:
-
Murine Embryonic Fibroblast
- HDF:
-
Human Dermal Fibroblasts
- ciPSCs:
-
Canine Induced Pluripotent Stem Cells
- imMKCLs:
-
Immortalize MK Progenitor Cell Line
- MK-FOP:
-
MK Forward Programming
- MMPs:
-
Matrix Metalloproteinase
- vWF:
-
Von Willebrand Factor
- GPIbα:
-
Platelet Glycoprotein Ibα
- GVHD:
-
Graft-versus-host disease
- B2M:
-
β2-Microglobulin
- KO:
-
Knockout
- CDC:
-
Cellular-dependent Cytotoxicity
- ADCC:
-
Antibody-dependent Cell-mediated Cytotoxicity
- FNAIT:
-
Fetal/neonatal Alloimmune Thrombocytopenia
- PTP:
-
Post-Transfusion Purpura
References
van der Meijden, P. E. J., & Heemskerk, J. W. M. (2019). Platelet biology and functions: New concepts and clinical perspectives. Nature Reviews. Cardiology, 16(3), 166–179.
Stanworth, S. J., et al. (2015). Platelet refractoriness–practical approaches and ongoing dilemmas in patient management. British Journal of Haematology, 171(3), 297–305.
Semple, J. W., Italiano, J. E., & Freedman, J. (2011). Platelets and the immune continuum. Nature Reviews Immunology, 11(4), 264–274.
Karagiannis, P., Sugimoto, N., & Eto, K. (2019). 66 - Stem Cell-Derived Platelets, in Platelets (Fourth Edition), A.D. Michelson, Editor. Academic Press. 1173–1189.
Dillard, G. H. L., Brecher, G., & Cronkite, E. P. (1951). Separation, Concentration, and Transfusion of Platelets. Proceedings of the Society for Experimental Biology and Medicine, 78(3), 796–799.
Szczepiorkowski, Z. M., & Dunbar, N. M. (2013). Transfusion guidelines: When to transfuse. Hematology. American Society of Hematology. Education Program, 2013, 638–644.
Etulain, J. (2018). Platelets in wound healing and regenerative medicine. Platelets, 29(6), 556–568.
Garbin, L. C., & Olver, C. S. (2020). Platelet-Rich Products and Their Application to Osteoarthritis. Journal of Equine Veterinary Science, 86:102820.
Rumjantseva, V., & Hoffmeister, K. M. (2010). Novel and unexpected clearance mechanisms for cold platelets. Transfusion and Apheresis Science, 42(1), 63–70.
Jenkins, C., et al. (2011). Bacterial contamination in platelets: Incremental improvements drive down but do not eliminate risk. Transfusion, 51(12), 2555–2565.
Brown, C. J., & Navarrete, C. V. (2011). Clinical relevance of the HLA system in blood transfusion. Vox Sanguinis, 101(2), 93–105.
Sugimoto, N., & Eto, K. (2017). Platelet production from induced pluripotent stem cells. Journal of Thrombosis and Haemostasis, 15(9), 1717–1727.
Avanzi, M. P., & Mitchell, W. B. (2014). Ex Vivo production of platelets from stem cells. British Journal of Haematology, 165(2), 237–247.
Karagiannis, P., Endo, H., & Eto, K. (2016). Generating Blood from iPS Cells. In H. Schulze & J. Italiano (Eds.), Molecular and Cellular Biology of Platelet Formation: Implications in Health and Disease (pp. 399–420). Springer International Publishing.
Mazur, E., et al. (1990). Isolation of large numbers of enriched human megakaryocytes from liquid cultures of normal peripheral blood progenitor cells. Blood, 76, 1771–1782.
Pineault, N., & Boisjoli, G. J. (2015). Megakaryopoiesis and ex vivo differentiation of stem cells into megakaryocytes and platelets. ISBT Science Series, 10(S1), 154–162.
Guerriero, R., et al. (2001). Stromal cell–derived factor 1α increases polyploidization of megakaryocytes generated by human hematopoietic progenitor cells. Blood, 97(9), 2587–2595.
De Bruyn, C., et al. (2005). Ex vivo expansion of megakaryocyte progenitor cells: Cord blood versus mobilized peripheral blood. Stem Cells Development, 14(4), 415–424.
Kawano, Y., et al. (2003). Ex vivo expansion of human umbilical cord hematopoietic progenitor cells using a coculture system with human telomerase catalytic subunit (hTERT)–transfected human stromal cells. Blood, 101(2), 532–540.
Matsunaga, T., et al. (2006). Ex Vivo Large-Scale Generation of Human Platelets from Cord Blood CD34+ Cells. Stem Cells, 24(12), 2877–2887.
Mostafa, S. S., Miller, W. M., & Papoutsakis, E. T. (2000). Oxygen tension influences the differentiation, maturation and apoptosis of human megakaryocytes. British Journal of Haematology, 111(3), 879–889.
Yang, H., Miller, W. M., & Papoutsakis, E. T. (2002). Higher pH promotes megakaryocytic maturation and apoptosis. Stem Cells, 20(4), 320–328.
Panuganti, S., et al. (2013). Three-stage ex vivo expansion of high-ploidy megakaryocytic cells: Toward large-scale platelet production. Tissue engineering. Part A, 19(7–8), 998–1014.
Yang, Y., et al. (2016). Integrated Biophysical and Biochemical Signals Augment Megakaryopoiesis and Thrombopoiesis in a Three-Dimensional Rotary Culture System. Stem Cells Translational Medicine, 5(2), 175–185.
Zhang, Y., et al. (2012). Small molecules, big roles – the chemical manipulation of stem cell fate and somatic cell reprogramming. Journal of Cell Science, 125(Pt 23), 5609–5620.
Li, W., et al. (2013). Chemical approaches to studying stem cell biology. Cell Research, 23(1), 81–91.
Yu, C., et al. (2014). Chemical approaches to cell reprogramming. Current Opinion in Genetics & Development, 28, 50–56.
Huang, N., et al. (2016). Identification of a potent small molecule capable of regulating polyploidization, megakaryocyte maturation, and platelet production. Journal of hematology & oncology, 9(1), 136–136.
Bhatlekar, S., et al. (2019). Anti-apoptotic BCL2L2 increases megakaryocyte proplatelet formation in cultures of human cord blood. Haematologica, 104(10), 2075–2083.
Nurhayati, R. W., Ojima, Y., & Taya, M. (2016). Recent developments in ex vivo platelet production. Cytotechnology, 68(6), 2211–2221.
Keller, G. M. (1995). In vitro differentiation of embryonic stem cells. Current Opinion in Cell Biology, 7(6), 862–869.
Gaur, M., et al. (2006). Megakaryocytes derived from human embryonic stem cells: A genetically tractable system to study megakaryocytopoiesis and integrin function. Journal of Thrombosis and Haemostasis, 4(2), 436–442.
Takayama, N., et al. (2008). Generation of functional platelets from human embryonic stem cells in vitro via ES-sacs, VEGF-promoted structures that concentrate hematopoietic progenitors. Blood, 111(11), 5298–5306.
Lu, S. J., et al. (2011). Platelets generated from human embryonic stem cells are functional in vitro and in the microcirculation of living mice. Cell Research, 21(3), 530–545.
Storm, M. P., et al. (2010). Three-dimensional culture systems for the expansion of pluripotent embryonic stem cells. Biotechnology and Bioengineering, 107(4), 683–695.
Lei, Y., & Schaffer, D. V. (2013). A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc Natl Acad Sci U S A, 110(52), E5039–E5048.
Zhang, B., et al. (2021). Large-scale generation of megakaryocytes from human embryonic stem cells using transgene-free and stepwise defined suspension culture conditions. Cell Prolife, 54(4):e13002.
Takahashi, K., & Yamanaka, S. (2006). Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell, 126(4), 663–676.
Takahashi, K., et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131(5), 861–872.
Shi, Y., et al. (2017). Induced pluripotent stem cell technology: A decade of progress. Nature reviews Drug discovery, 16(2), 115–130.
Moradi, S., et al. (2019). Research and therapy with induced pluripotent stem cells (iPSCs): Social, legal, and ethical considerations. Stem Cell Research & Therapy, 10(1), 341.
Li, F., Hu, J., & He, T. C. (2017). iPSC-based treatment of age-related macular degeneration (AMD): The path to success requires more than blind faith. Genes Dis, 4(2), 41–42.
Takahashi, J. (2020). iPS cell-based therapy for Parkinson’s disease: A Kyoto trial. Regen Ther, 13, 18–22.
Turner, D., et al. (2020). Clinical-based Cell Therapies for Heart Disease-Current and Future State. Rambam Maimonides Medical Journal, 11(2):e0015.
Yamanaka, S. (2012). Induced Pluripotent Stem Cells: Past, Present, and Future. Cell Stem Cell, 10(6), 678–684.
Yu, J., et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science, 318(5858), 1917–1920.
Montserrat, N., et al. (2013). Reprogramming of Human Fibroblasts to Pluripotency with Lineage Specifiers. Cell Stem Cell, 13(3), 341–350.
Stadtfeld, M., et al. (2008). Induced pluripotent stem cells generated without viral integration. Science, 322(5903), 945–949.
Fusaki, N., et al. (2009). Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proceedings of the Japan Academy, Series B, 85(8), 348–362.
Woltjen, K., et al. (2009). piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature, 458(7239), 766–770.
Worringer, Kathleen A., et al. (2014). The let-7/LIN-41 Pathway Regulates Reprogramming to Human Induced Pluripotent Stem Cells by Controlling Expression of Prodifferentiation Genes. Cell Stem Cell, 14(1):40–52.
Esteban, M. A., et al. (2010). Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell, 6(1), 71–79.
Hou, P., et al. (2013). Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science, 341(6146), 651–654.
Warren, L., et al. (2010). Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell, 7(5), 618–630.
Kim, D., et al. (2009). Generation of Human Induced Pluripotent Stem Cells by Direct Delivery of Reprogramming Proteins. Cell Stem Cell, 4(6), 472–476.
Musunuru, K., et al. (2018). Induced Pluripotent Stem Cells for Cardiovascular Disease Modeling and Precision Medicine: A Scientific Statement From the American Heart Association. Circulation Genomic and Precison Medicine, 11(1):e000043.
Liu, Y., et al. (2012). The gene expression profiles of induced pluripotent stem cells (iPSCs) generated by a non-integrating method are more similar to embryonic stem cells than those of iPSCs generated by an integrating method. Genetics and molecular biology, 35(3), 693–700.
Mandai, M., et al. (2017). Autologous Induced Stem-Cell–Derived Retinal Cells for Macular Degeneration. New England Journal of Medicine, 376(11), 1038–1046.
Gekas, C., & Graf, T. (2010). Induced pluripotent stem cell-derived human platelets: One step closer to the clinic. The Journal of experimental medicine, 207(13), 2781–2784.
Takayama, N., et al. (2010). Transient activation of c-MYC expression is critical for efficient platelet generation from human induced pluripotent stem cells. The Journal of experimental medicine, 207(13), 2817–2830.
Nishimura, T., et al. (2013). Generation of functional platelets from canine induced pluripotent stem cells. Stem Cells Dev, 22(14), 2026–2035.
Feng, Q., et al. (2014). Scalable Generation of Universal Platelets from Human Induced Pluripotent Stem Cells. Stem Cell Reports, 3(5), 817–831.
Liu, Y., et al. (2015). Efficient generation of megakaryocytes from human induced pluripotent stem cells using food and drug administration-approved pharmacological reagents. Stem cells translational medicine, 4(4), 309–319.
Rodin, S., et al. (2010). Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nature Biotechnology, 28(6), 611–615.
Mei, Y., et al. (2010). Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nature Materials, 9(9), 768–778.
Lee, H., et al. (2016). Establishment of feeder-free culture system for human induced pluripotent stem cell onDAS nanocrystalline graphene. Scientific Reports, 6(1), 20708.
Nakamura, S., et al. (2014). Expandable megakaryocyte cell lines enable clinically applicable generation of platelets from human induced pluripotent stem cells. Cell Stem Cell, 14(4), 535–548.
Moreau, T., et al. (2016). Large-scale production of megakaryocytes from human pluripotent stem cells by chemically defined forward programming. Nature Communications, 7, 11208.
Ingavle, G., et al. (2019). Mimicking megakaryopoiesis in vitro using biomaterials: Recent advances and future opportunities. Acta Biomaterialia, 96, 99–110.
Thon, J. N., et al. (2014). Platelet bioreactor-on-a-chip. Blood, 124(12), 1857–1867.
Blin, A., et al. (2016). Microfluidic model of the platelet-generating organ: Beyond bone marrow biomimetics. Scientific Reports, 6(1), 21700.
Ito, Y., et al. (2018). Turbulence Activates Platelet Biogenesis to Enable Clinical Scale Ex Vivo Production. Cell, 174(3), 636-648.e18.
Hosseini, E., Mohtashami, M., & Ghasemzadeh, M. (2019). Down-regulation of platelet adhesion receptors is a controlling mechanism of thrombosis, while also affecting post-transfusion efficacy of stored platelets. Thrombosis Journal, 17(1), 20.
Hirata, S., et al. (2017). Selective Inhibition of ADAM17 Efficiently Mediates Glycoprotein Ibα Retention During Ex Vivo Generation of Human Induced Pluripotent Stem Cell-Derived Platelets. Stem cells translational medicine, 6(3), 720–730.
Ono, Y., et al. (2012). Induction of functional platelets from mouse and human fibroblasts by p45NF-E2/Maf. Blood, 120(18), 3812–3821.
Pulecio, J., et al. (2016). Direct Conversion of Fibroblasts to Megakaryocyte Progenitors. Cell Reports, 17(3), 671–683.
Madrid, M., et al. (2021). Autologous Induced Pluripotent Stem Cell–Based Cell Therapies: Promise, Progress, and Challenges. Current Protocols, 1(3):e88.
Basire, A., & Picard, C. (2014). Platelet allo-antibodies identification strategies for preventing and managing platelet refractoriness. Transfusion Clinique et Biologique, 21(4–5), 193–206.
Heal, J. M., et al. (1993). The role of ABO matching in platelet transfusion. European Journal of Haematology, 50(2), 110–117.
Estcourt, L. J., et al. (2017). Guidelines for the use of platelet transfusions. British Journal of Haematology, 176(3), 365–394.
Gourraud, P.-A., et al. (2012). The Role of Human Leukocyte Antigen Matching in the Development of Multiethnic “Haplobank” of Induced Pluripotent Stem Cell Lines. STEM CELLS, 30(2), 180–186.
Figueiredo, C., & Blasczyk, R. (2021). Generation of HLA Universal Megakaryocytes and Platelets by Genetic Engineering. Frontiers in Immunology, 12.
Börger, A. K., et al. (2016). Generation of HLA-Universal iPSC-Derived Megakaryocytes and Platelets for Survival Under Refractoriness Conditions. Molecular Medicine, 22, 274–285.
Xu, H., et al. (2019). Targeted Disruption of HLA Genes via CRISPR-Cas9 Generates iPSCs with Enhanced Immune Compatibility. Cell Stem Cell, 24(4), 566-578.e7.
Saito, S., et al. (2002). Platelet transfusion refractoriness caused by a mismatch in HLA-C antigens. Transfusion, 42(3), 302–308.
Suzuki, D., et al. (2019). iPSC-Derived Platelets Depleted of HLA Class I Are Inert to Anti-HLA Class I and Natural Killer Cell Immunity. Stem Cell Reports.
Gopalappa, R., et al. (2018). Paired D10A Cas9 nickases are sometimes more efficient than individual nucleases for gene disruption. Nucleic Acids Research, 46(12), e71–e71.
Ran, F. A., et al. (2013). Double Nicking by RNA-Guided CRISPR Cas9 for Enhanced Genome Editing Specificity. Cell, 154(6), 1380–1389.
Norbnop, P., et al. (2020). Generation and characterization of HLA-universal platelets derived from induced pluripotent stem cells. Scientific reports, 10(1), 8472–8472.
Zhang, N., et al. (2016). CRISPR/Cas9-mediated conversion of human platelet alloantigen allotypes. Blood, 127(6), 675–680.
Acknowledgements
We acknowledge the staffs of Pediatric Cell and Gene Therapy Research Center, Gene, Cell & Tissue Research Institute, Tehran university of medical sciences, Tehran, Iran.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
EI and ZS were contributed in paper writing and generated figures. AAH were contributed in data discussion and revised the manuscript LPL and AA were contributed in revised the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent for Publication
Not applicable.
Competing Interest
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Izady, E., Saltanatpour, Z., Liu, LP. et al. Toward in Vitro Production of Platelet from Induced Pluripotent Stem Cells. Stem Cell Rev and Rep 18, 2376–2387 (2022). https://doi.org/10.1007/s12015-022-10366-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10366-4