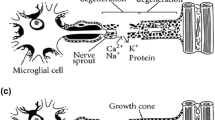
Abstract
Peripheral nerve injuries (PNIs) are common and debilitating, cause significant health care costs for society, and rely predominately on autografts, which necessitate grafting a nerve section non-locally to repair the nerve injury. One possible approach to improving treatment is bolstering endogenous regenerative mechanisms or bioengineering new nervous tissue in the peripheral nervous system. In this review, we discuss critical-sized nerve gaps and nerve regeneration in rats, and summarize the roles of adipose-derived stem cells (ADSCs) in the treatment of PNIs. Several regenerative treatment modalities for PNI are described: ADSCs differentiating into Schwann cells (SCs), ADSCs secreting growth factors to promote peripheral nerve growth, ADSCs promoting myelination growth, and ADSCs treatments with scaffolds. ADSCs’ roles in regenerative treatment and features are compared to mesenchymal stem cells, and the administration routes, cell dosages, and cell fates are discussed. ADSCs secrete neurotrophic factors and exosomes and can differentiate into Schwann cell-like cells (SCLCs) that share features with naturally occurring SCs, including the ability to promote nerve regeneration in the PNS. Future clinical applications are also discussed.
Graphical abstract


Similar content being viewed by others
Abbreviations
- PNIs:
-
Peripheral nerve injuries
- ADSCs:
-
Adipose-derived stem cells
- uADSCs:
-
Undifferentiated ADSCs
- dADSCs:
-
Differentiated ADSCs
- SCs:
-
Schwann cells
- SCLCs:
-
Schwann cell-like cells
- NGCs:
-
Neural guidance conduits
- MSCs:
-
Mesenchymal stem cells
- BMMSCs:
-
Bone marrow mesenchymal stem cells
References
Jiang, L., Jones, S., & Jia, X. (2017). Stem cell transplantation for peripheral nerve regeneration: Current options and opportunities. International Journal of Molecular Science, 18, 1.
Jones, S., Eisenberg, H. M., & Jia, X. (2016). Advances and Future applications of augmented peripheral nerve regeneration. International Journal of Molecular Science, 17, 9.
Hallgren, A., et al. (2013). Subjective outcome related to donor site morbidity after sural nerve graft harvesting: A survey in 41 patients. BMC Surgery, 13, 39.
Kehoe, S., Zhang, X. F., & Boyd, D. (2012). FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury, 43(5), 553–572.
Deumens, R., et al. (2010). Repairing injured peripheral nerves: Bridging the gap. Progress in Neurobiology, 92(3), 245–276.
Guntinas-Lichius, O., et al. (2005). Factors limiting motor recovery after facial nerve transection in the rat: Combined structural and functional analyses. European Journal of Neuroscience, 21(2), 391–402.
Boriani, F., et al. (2017). A novel technique for decellularization of allogenic nerves and in vivo study of their use for peripheral nerve reconstruction. Journal of Biomedical Materials and Research A, 105(8), 2228–2240.
Willand, M. P., et al. (2016). Electrical stimulation to promote peripheral nerve regeneration. Neurorehabilitation and Neural Repair, 30(5), 490–496.
Kaplan, H. M., Mishra, P., & Kohn, J. (2015). The overwhelming use of rat models in nerve regeneration research may compromise designs of nerve guidance conduits for humans. Journal of Materials Science: Materials in Medicine, 26(8), 226.
Georgiou, M., et al. (2015). Engineered neural tissue with aligned, differentiated adipose-derived stem cells promotes peripheral nerve regeneration across a critical sized defect in rat sciatic nerve. Biomaterials, 37, 242–251.
Gaudin, R., et al. (2016). Approaches to peripheral nerve repair: Generations of biomaterial conduits yielding to replacing autologous nerve grafts in craniomaxillofacial surgery. Biomed Research International, 2016, 3856262.
Labroo, P., et al. (2019). Drug-delivering nerve conduit improves regeneration in a critical-sized gap. Biotechnology and Bioengineering, 116(1), 143–154.
Berrocal, Y. A., et al. (2013). Transplantation of Schwann cells in a collagen tube for the repair of large, segmental peripheral nerve defects in rats. Journal of Neurosurgery, 119(3), 720–732.
Ursu, D., et al. (2017). Adjacent regenerative peripheral nerve interfaces produce phase-antagonist signals during voluntary walking in rats. Journal of Neuroengineering Rehabilation, 14(1), 33.
Xu, F., et al. (2017). NECL1 coated PLGA as favorable conduits for repair of injured peripheral nerve. Materials Science and Engineering C: Materials in Biology Application, 70(Pt 2), 1132–1140.
Lewitus, D., et al. (2011). Designing tyrosine-derived polycarbonate polymers for biodegradable regenerative type neural interface capable of neural recording. IEEE Transaction on Neural System Rehabilation Engineering, 19(2), 204–212.
Sullivan, R., et al. (2016). Peripheral nerve injury: Stem cell therapy and peripheral nerve transfer. International Journal of Molecular Science, 17, 12.
Sun, X., et al. (2018). Differentiation of adipose-derived stem cells into Schwann cell-like cells through intermittent induction: Potential advantage of cellular transient memory function. Stem Cell Research Theraphy, 9(1), 133.
Walocko, F. M., et al. (2016). The potential roles for adipose tissue in peripheral nerve regeneration. Microsurgery, 36(1), 81–88.
Houschyar, K. S., et al. (2016). The role of current techniques and concepts in peripheral nerve repair. Plastic Surgery International, 2016, 4175293.
Du, J., et al. (2018). Biomimetic neural scaffolds: A crucial step towards optimal peripheral nerve regeneration. Biomaterials and Science, 6(6), 1299–1311.
Du, J., et al. (2018). Optimal electrical stimulation boosts stem cell therapy in nerve regeneration. Biomaterials, 181, 347–359.
Burnett, M. G., & Zager, E. L. (2004). Pathophysiology of peripheral nerve injury: A brief review. Neurosurgery Focus, 16(5), E1.
Menorca, R. M., Fussell, T. S., & Elfar, J. C. (2013). Nerve physiology: Mechanisms of injury and recovery. Hand Clinic, 29(3), 317–330.
Girard, C., et al. (2008). Etifoxine improves peripheral nerve regeneration and functional recovery. Proceedings of the Natational Academic Science USA, 105(51), 20505–20510.
Oh, S. H., et al. (2008). Peripheral nerve regeneration within an asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. Biomaterials, 29(11), 1601–1609.
Young, R. C., Wiberg, M., & Terenghi, G. (2002). Poly-3-hydroxybutyrate (PHB): A resorbable conduit for long-gap repair in peripheral nerves. British Journal of Plastic Surgery, 55(3), 235–240.
Fawcett, J. W., & Keynes, R. J. (1986). Muscle basal lamina: A new graft material for peripheral nerve repair. Journal of Neurosurgery, 65(3), 354–363.
Humphrey, M. F., et al. (1989). Peripheral nerve repair across a gap studied by repeated observation in a new window implant chamber. Brain Research, 497(1), 132–137.
Park, S. C., et al. (2010). Ultrasound-stimulated peripheral nerve regeneration within asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. Journal of Biomedical Material Research B Applied Biomaterials, 94(2), 359–366.
Lopez-Verrilli, M. A., Picou, F., & Court, F. A. (2013). Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia, 61(11), 1795–1806.
Yuan, Y. C., et al. (2005). Axon and schwann cell partnership during nerve regrowth. Journal of Neuropathology and Experimental Neurology, 64(7), 613–622.
Kaewkhaw, R., Scutt, A. M., & Haycock, J. W. (2012). Integrated culture and purification of rat Schwann cells from freshly isolated adult tissue. Nature of Protocol, 7(11), 1996–2004.
Levi, A. D., et al. (2016). The use of autologous schwann cells to supplement sciatic nerve repair with a large gap: First in human experience. Cell Transplantation, 25(7), 1395–1403.
Rutkowski, J. L., et al. (1995). Purification and expansion of human Schwann cells in vitro. Nature of Medicine, 1(1), 80–83.
Kim, H. S., et al. (2017). Schwann cell precursors from human pluripotent stem cells as a potential therapeutic target for myelin repair. Stem Cell Reports, 8(6), 1714–1726.
Palomo Irigoyen, M., et al. (2018). Isolation and purification of primary rodent schwann cells. Methods Molecular Biology, 1791, 81–93.
Weiss, T., et al. (2018). Detailed protocols for the isolation, culture, enrichment and immunostaining of primary human Schwann cells. Methods Molecular Biology, 1739, 67–86.
Anderson, K. D., et al. (2017). Safety of autologous human Schwann cell transplantation in subacute thoracic spinal cord injury. Journal of Neurotrauma, 34(21), 2950–2963.
Reichenberger, M. A., et al. (2016). ADSCs in a fibrin matrix enhance nerve regeneration after epineural suturing in a rat model. Microsurgery, 36(6), 491–500.
Hsieh, S. C., et al. (2016). Effect of an epineurial-like biohybrid nerve conduit on nerve regeneration. Cell Transplantation, 25(3), 559–574.
He, X., et al. (2016). Transplantation of miRNA-34a overexpressing adipose-derived stem cell enhances rat nerve regeneration. Wound Repair and Regeneration, 24(3), 542–550.
Dezawa, M., et al. (2001). Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. European Journal of Neuroscience, 14(11), 1771–1776.
Lavorato, A., et al. (2021). Mesenchymal stem cell treatment perspectives in peripheral nerve regeneration: systematic review. International Journal of Molecular Science, 22, 2.
Zuk, P. A., et al. (2001). Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Engineering, 7(2), 211–228.
Gimble, J. M., Katz, A. J., & Bunnell, B. A. (2007). Adipose-derived stem cells for regenerative medicine. Circulation Research, 100(9), 1249–1260.
Zhou, L. N., et al. (2020). A comparison of the use of adipose-derived and bone marrow-derived stem cells for peripheral nerve regeneration in vitro and in vivo. Stem Cell Research Theraphy, 11(1), 153.
Beane, O. S., et al. (2014). Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS ONE, 9(12), e115963.
Rodriguez Sanchez, D. N., et al. (2019). Canine adipose-derived mesenchymal stromal cells enhance neuroregeneration in a rat model of sciatic nerve crush injury. Cell Transplantation, 28(1), 47–54.
Tremp, M., et al. (2018). Regeneration of nerve crush injury using adipose-derived stem cells: A multimodal comparison. Muscle and Nerve, 58(4), 566–572.
Kappos, E. A., et al. (2018). Epineural adipose-derived stem cell injection in a sciatic rodent model. Brain Behaviour, 8(7), e01027.
Kappos, E. A., et al. (2015). Peripheral nerve repair: Multimodal comparison of the long-term regenerative potential of adipose tissue-derived cells in a biodegradable conduit. Stem Cells and Development, 24(18), 2127–2141.
Li, Y. C., et al. (2014). A neural stem/precursor cell monolayer for neural tissue engineering. Biomaterials, 35(4), 1192–1204.
Lasso, J. M., et al. (2015). Xenotransplantation of human adipose-derived stem cells in the regeneration of a rabbit peripheral nerve. Journal of Plastic Reconstruction Aesthetics Surgery, 68(12), e189–e197.
Luo, H., et al. (2015). Tissue-engineered nerve constructs under a microgravity system for peripheral nerve regeneration. Tissue Engineering Part A, 21(1–2), 267–276.
Allbright, K. O., et al. (2018). Delivery of adipose-derived stem cells in poloxamer hydrogel improves peripheral nerve regeneration. Muscle and Nerve, 58(2), 251–260.
Santiago, L. Y., et al. (2009). Delivery of adipose-derived precursor cells for peripheral nerve repair. Cell Transplantation, 18(2), 145–158.
Klein, S. M., et al. (2016). Peripheral motor and sensory nerve conduction following transplantation of undifferentiated autologous adipose tissue-derived stem cells in a biodegradable US food and drug administration-approved nerve conduit. Plastic Reconstruction Surgery, 138(1), 132.
di Summa, P. G., et al. (2010). Adipose-derived stem cells enhance peripheral nerve regeneration. Journal of Plastic, Reconstructive and Aesthetic Surgery: JPRAS, 63(9), 1544–1552.
Kim, D. Y., et al. (2014). In vivo effects of adipose-derived stem cells in inducing neuronal regeneration in Sprague-Dawley rats undergoing nerve defect bridged with polycaprolactone nanotubes. Journal of Korean Medical Science, 29, S183–S192.
Han, I. H., et al. (2015). Cultures of Schwann-like cells differentiated from adipose-derived stem cells on PDMS/MWNT sheets as a scaffold for peripheral nerve regeneration. Journal of Biomedical Materials Research Part A, 103(11), 3642–3648.
Kolar, M. K., & Kingham, P. J. (2014). Regenerative effects of adipose-tissue-derived stem cells for treatment of peripheral nerve injuries. Biochemical Society Transactions, 42(3), 697–701.
Fu, X. M., et al. (2019). The combination of adipose-derived schwann-like cells and acellular nerve allografts promotes sciatic nerve regeneration and repair through the JAK2/STAT3 signaling pathway in rats. Neuroscience, 422, 134–145.
Marconi, S., et al. (2012). Human adipose-derived mesenchymal stem cells systemically injected promote peripheral nerve regeneration in the mouse model of sciatic crush. Tissue Engineering Part A, 18(11–12), 1264–1272.
Schweizer, R., et al. (2020). Effect of Systemic adipose-derived stem cell therapy on functional nerve regeneration in a rodent model. Plastic Reconstruction Surgery Global Open, 8(7), e2953.
Lin, G., et al. (2011). Tracking intracavernously injected adipose-derived stem cells to bone marrow. International Journal of Impotence Research, 23(6), 268–275.
Rbia, N., et al. (2018). A simple dynamic strategy to deliver stem cells to decellularized nerve allografts. Plastic Reconstruction Surgery, 142(2), 402–413.
Wang, D., Wang, S., & Shi, C. (2012). Update on cancer related issues of mesenchymal stem cell-based therapies. Current Stem Cell and Reserach Therapy, 7(5), 370–380.
Lee, A. S., et al. (2013). Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Natural Medicine, 19(8), 998–1004.
Wu, H., et al. (2018). Nanotechnology-assisted adipose-derived stem cell (ADSC) therapy for erectile dysfunction of cavernous nerve injury. Asian Journal of Andrology, 20(5), 442–447.
Burdick, J. A., Mauck, R. L., & Gerecht, S. (2016). To serve and protect: Hydrogels to improve stem cell-based therapies. Cell Stem Cell, 18(1), 13–15.
Sart, S., Ma, T., & Li, Y. (2014). Preconditioning stem cells for in vivo delivery. Bioresearch Open Access, 3(4), 137–149.
Hwang, D. W., et al. (2014). In vivo bioluminescence imaging for prolonged survival of transplanted human neural stem cells using 3D biocompatible scaffold in corticectomized rat model. PLoS ONE, 9(9), e105129.
Hyun, J. S., et al. (2013). Enhancing stem cell survival in vivo for tissue repair. Biotechnology Advance, 31(5), 736–743.
Masgutov, R., et al. (2018). Allogenic adipose derived stem cells transplantation improved sciatic nerve regeneration in rats: Autologous nerve graft model. Frontier Pharmacology, 9, 86.
Rbia, N., et al. (2019). In vivo survival of mesenchymal stromal cell-enhanced decellularized nerve grafts for segmental peripheral nerve reconstruction. The Journal of Hand Surgery, 44(6), 514.e1-514.e11.
Meyerrose, T. E., et al. (2007). In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells, 25(1), 220–227.
Erba, P., et al. (2010). Regeneration potential and survival of transplanted undifferentiated adipose tissue-derived stem cells in peripheral nerve conduits. Journal of Plastic Reconstruction Aesthetics Surgery, 63(12), e811–e817.
Wolbank, S., et al. (2007). Labelling of human adipose-derived stem cells for non-invasive in vivo cell tracking. Cell and Tissue Banking, 8(3), 163–177.
Lequeux, C., et al. (2011). Adipose derived stem cells: Efficiency, toxicity, stability of BrdU labeling and effects on self-renewal and adipose differentiation. Molecular Cell Biochemistry, 351(1–2), 65–75.
Orbay, H., et al. (2012). Differentiated and undifferentiated adipose-derived stem cells improve function in rats with peripheral nerve gaps. Journal of Plastic, Reconstructive and Aesthetic Surgery, 65(5), 657–664.
Liao, D., et al. (2010). Co-culture with Schwann cells is an effective way for adipose-derived stem cells neural transdifferentiation. Archies Medical Science, 6(2), 145–151.
Tomita, K., et al. (2013). Glial differentiation of human adipose-derived stem cells: Implications for cell-based transplantation therapy. Neuroscience, 236, 55–65.
Faroni, A., et al. (2013). Differentiation of adipose-derived stem cells into Schwann cell phenotype induces expression of P2X receptors that control cell death. Cell Death Diseases, 4, e743.
Walsh, S. K., et al. (2012). Fate of stem cell transplants in peripheral nerves. Stem Cell Research, 8(2), 226–238.
Walsh, S., & Midha, R. (2009). Practical considerations concerning the use of stem cells for peripheral nerve repair. Neurosurgical Focus, 26(2), E2.
Abbas, O. L., et al. (2016). Adipose-derived stem cells enhance axonal regeneration through cross-facial nerve grafting in a rat model of facial paralysis. Plastic and Reconstructive Surgery, 138(2), 387–396.
Guo, J., et al. (2017). Promoting potential of adipose derived stem cells on peripheral nerve regeneration. Molecular Medicine Reports, 16(5), 7297–7304.
Xu, Y., et al. (2008). Myelin-forming ability of Schwann cell-like cells induced from rat adipose-derived stem cells in vitro. Brain Research, 1239, 49–55.
Wei, Y., et al. (2010). Schwann-like cell differentiation of rat adipose-derived stem cells by indirect co-culture with Schwann cells in vitro. Cell Proliferation, 43(6), 606–616.
Jiang, L., et al. (2008). Differentiation of rat adipose tissue-derived stem cells into Schwann-like cells in vitro. NeuroReport, 19(10), 1015–1019.
Kingham, P. J., et al. (2007). Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Experimental Neurology, 207(2), 267–274.
Xu, Y., et al. (2008). Neurospheres from rat adipose-derived stem cells could be induced into functional Schwann cell-like cells in vitro. BMC Neuroscience, 9, 21.
Gao, S., et al. (2015). Different methods for inducing adipose-derived stem cells to differentiate into Schwann-like cells. Archives Medical Sciences, 11(4), 886–892.
Younesi, E., et al. (2015). Differentiation of adipose-derived stem cells into Schwann-like cells: Fetal bovine serum or human serum? Anatomy and Cell Biology, 48(3), 170–176.
Ladak, A., et al. (2011). Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Experimental Neurology, 228(2), 242–252.
Hou, S. Y., et al. (2006). Tissue-engineered peripheral nerve grafting by differentiated bone marrow stromal cells. Neuroscience, 140(1), 101–110.
Tse, K. H., et al. (2015). Intrinsic mechanisms underlying the neurotrophic activity of adipose derived stem cells. Experimental Cell Research, 331(1), 142–151.
Piovesana, R., et al. (2019). M2 receptors activation modulates cell growth, migration and differentiation of rat Schwann-like adipose-derived stem cells. Cell Death Discovery, 5, 92.
Kingham, P. J., Mantovani, C., & Terenghi, G. (2009). Notch independent signalling mediates Schwann cell-like differentiation of adipose derived stem cells. Neuroscience Letter, 467(2), 164–168.
Faroni, A., et al. (2016). Human Schwann-like cells derived from adipose-derived mesenchymal stem cells rapidly de-differentiate in the absence of stimulating medium. European Journal of Neurosciences, 43(3), 417–430.
Mortimer, A. E., et al. (2017). Maintenance of a Schwann-like phenotype in differentiated adipose-derived stem cells requires the synergistic action of multiple growth factors. Stem Cells and International, 2017, 1479137.
Moosazadeh Moghaddam, M., et al. (2019). Engineered substrates with imprinted cell-like topographies induce direct differentiation of adipose-derived mesenchymal stem cells into Schwann cells. Artificial Cells Nanomedicine and Biotechnology, 47(1), 1022–1035.
Kingham, P. J., et al. (2014). Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Development, 23(7), 741–754.
Tomita, K., et al. (2012). Differentiated adipose-derived stem cells promote myelination and enhance functional recovery in a rat model of chronic denervation. Journal of Neuroscience Research, 90(7), 1392–1402.
Scholz, T., et al. (2011). Neuronal differentiation of human adipose tissue–derived stem cells for peripheral nerve regeneration in vivostem cells for peripheral nerve regeneration. Archives of Surgery, 146(6), 666–674.
Gao, S., et al. (2014). Differentiation of human adipose-derived stem cells into neuron-like cells which are compatible with photocurable three-dimensional scaffolds. Tissue Engineering Part A, 20(7–8), 1271–1284.
Watanabe, Y., et al. (2017). Undifferentiated and differentiated adipose-derived stem cells improve nerve regeneration in a rat model of facial nerve defect. Journal of Tissue Engineering and Regenerative Medicine, 11(2), 362–374.
Mathot, F., et al. (2020). Gene expression profiles of differentiated and undifferentiated adipose derived mesenchymal stem cells dynamically seeded onto a processed nerve allograft. Gene, 724, 144151.
Di Summa, P. G., et al. (2018). Adipose derived stem cells reduce fibrosis and promote nerve regeneration in rats. The Anatomical Record (Hoboken), 301(10), 1714–1721.
Sowa, Y., et al. (2016). Adipose-derived stem cells promote peripheral nerve regeneration in vivo without differentiation into Schwann-like lineage. Plastic Reconstruction Surgery, 137(2), 318e–330e.
Zuk, P. (2013). Adipose-derived stem cells in tissue regeneration: A review. ISRN Stem Cells, 2013, 1–35.
Kalbermatten, D. F., et al. (2011). Neurotrophic activity of human adipose stem cells isolated from deep and superficial layers of abdominal fat. Cell Tissue Research, 344(2), 251–260.
Wilkins, A., et al. (2009). Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Research, 3(1), 63–70.
Taghi, G. M., et al. (2012). Characterization of in vitro cultured bone marrow and adipose tissue-derived mesenchymal stem cells and their ability to express neurotrophic factors. Cell Biology International, 36(12), 1239–1249.
Osugi, M., et al. (2012). Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Engineering Part A, 18(13–14), 1479–1489.
Kaewkhaw, R., Scutt, A. M., & Haycock, J. W. (2011). Anatomical site influences the differentiation of adipose-derived stem cells for Schwann-cell phenotype and function. Glia, 59(5), 734–749.
Engels, P. E., et al. (2013). Harvest site influences the growth properties of adipose derived stem cells. Cytotechnology, 65(3), 437–445.
Mathias, T., et al. (2015). The regeneration potential after human and autologous stem cell transplantation in a rat sciatic nerve injury model can be monitored by MRI. Cell Transplation, 24(2), 203–211.
Mantovani, C., et al. (2012). Morphological, molecular and functional differences of adult bone marrow- and adipose-derived stem cells isolated from rats of different ages. Experimental Cell, 318(16), 2034–2048.
Yoshihiro, S., et al. (2012). Adipose-derived stem cells produce factors enhancing peripheral nerve regeneration: Influence of age and anatomic site of origin. Stem Cells and Development, 21(11), 1852–1862.
Kingham, J. P., et al. (2014). Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells and Development, 23(7), 741–754.
Ochoa, J., et al. (1971). Nature of the nerve lesion caused by a pneumatic tourniquet. Nature, 233(5317), 265–266.
Masgutov, R. F., et al. (2016). Human adipose-derived stem cells stimulate neuroregeneration. Clinical Experimental Medicine, 16(3), 451–461.
Xie, S., et al. (2017). Efficient generation of functional Schwann cells from adipose-derived stem cells in defined conditions. Cell Cycle, 16(9), 841–851.
Qing, L., et al. (2018). Exosomes and their MicroRNA cargo: New players in peripheral nerve regeneration. Neurorehabiliation Neural Repair, 32(9), 765–776.
Bucan, V., et al. (2019). Effect of exosomes from rat adipose-derived mesenchymal stem cells on neurite outgrowth and sciatic nerve regeneration after crush injury. Molecular Neurobiology, 56(3), 1812–1824.
Ching, R. C., Wiberg, M., & Kingham, P. J. (2018). Schwann cell-like differentiated adipose stem cells promote neurite outgrowth via secreted exosomes and RNA transfer. Stem Cells and Research Theraphy, 9(1), 266.
Liu, C. Y., et al. (2019). Effect of exosomes from adipose-derived stem cells on the apoptosis of Schwann cells in peripheral nerve injury. CNS Neuroscience and Theraphy.
Farinazzo, A., et al. (2015). Murine adipose-derived mesenchymal stromal cell vesicles: In vitro clues for neuroprotective and neuroregenerative approaches. Cytotherapy, 17(5), 571–578.
Jacob, J. M., & Robbins, N. (1990). Differential effects of age on neuromuscular transmission in partially denervated mouse muscle. Journal of Neuroscience the Official Journal of the Society for Neuroscience, 10(5), 1522.
Syu, W. Z., et al. (2019). Adipose-derived neural stem cells combined with acellular dermal matrix as a neural conduit enhances peripheral nerve repair. Cell Transplantation, 28(9–10), 1220–1230.
Kim, Y. M., et al. (2009). Effects of systemic transplantation of adipose tissue-derived stem cells on olfactory epithelium regeneration. The Laryngoscope, 119(5), 993–999.
Park, J. U., & Kwon, S. T. (2017). Potential of autologous adipose-derived stem cells to regenerate atrophied muscle in a rat model. Wound Repair Regeneration, 25(6), 944–955.
Rbia, N., et al. (2019). In vivo survival of mesenchymal stromal cell-enhanced decellularized nerve grafts for segmental peripheral nerve reconstruction. Journal of Hand Surgery American, 44(6), 514.
Funding
XJ was supported in part by Maryland Stem Cell Research Fund, USA (2018-MSCRFD-4271 and 2020-MSCRFD-5384) (both to XJ), NIH RO1 NS117102 (to XJ).
Author information
Authors and Affiliations
Contributions
LJ contributed with data acquisition and analysis, searched and reviewed the literature, drafted and revised the manuscript; XZ searched and reviewed the literature, and drafted the manuscript; TM reviewed the literature and revised the manuscript; XJ conceived the idea, designed and formulated the review theme, viewed the literature, data analysis, and revised and finalized the manuscript.
Corresponding author
Ethics declarations
Conflict Interest
The authors declared no potential conflicts of interest. The founding sponsors had no role in the writing of this review.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance statement: This concise review seeks to highlight the recent advances in augmenting nerve regeneration after peripheral nerve injury using adipose-derived stem cells with a focus on administration routes, cell dosages, cell fates, and underlying therapeutic mechanisms.
Rights and permissions
About this article
Cite this article
Jiang, L., Mee, T., Zhou, X. et al. Augmenting Peripheral Nerve Regeneration with Adipose-Derived Stem Cells. Stem Cell Rev and Rep 18, 544–558 (2022). https://doi.org/10.1007/s12015-021-10236-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-021-10236-5