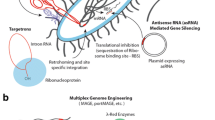
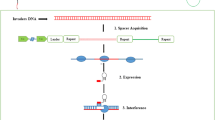
Abstract
In recent decades, the development of novel antimicrobials has significantly slowed due to the emergence of antimicrobial resistance (AMR), intensifying the global struggle against infectious diseases. Microbial populations worldwide rapidly develop resistance due to the widespread use of antibiotics, primarily targeting drug-resistant germs. A prominent manifestation of this resistance is the formation of biofilms, where bacteria create protective layers using signaling pathways such as quorum sensing. In response to this challenge, the CRISPR-Cas9 method has emerged as a ground-breaking strategy to counter biofilms. Initially identified as the “adaptive immune system” of bacteria, CRISPR-Cas9 has evolved into a state-of-the-art genetic engineering tool. Its exceptional precision in altering specific genes across diverse microorganisms positions it as a promising alternative for addressing antibiotic resistance by selectively modifying genes in diverse microorganisms. This comprehensive review concentrates on the historical background, discovery, developmental stages, and distinct components of CRISPR Cas9 technology. Emphasizing its role as a widely used genome engineering tool, the review explores how CRISPR Cas9 can significantly contribute to the targeted disruption of genes responsible for biofilm formation, highlighting its pivotal role in reshaping strategies to combat antibiotic resistance and mitigate the challenges posed by biofilm-associated infectious diseases.






Similar content being viewed by others
References
Rice, L. B. (2008). Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. The Journal of Infectious Diseases, 197(8), 1079–1081.
Pineda Solas, V., Perez Benito, A., Domingo Puiggros, M., Larramona Carrera, H., Segura Porta, F. & & Fontanals Aymerich, D. (2002). Bacteremic pneumococcal pneumonia. Anales Espanoles de Pediatria, 57(5), 408–413.
Hawkey, P. M. (2015). Multi-drug resistant Gram-negative bacteria: a product of globalization. Journal of Hospital Infection, 89, 241–247.
Aslam, B., Wang, W., Arshad, M. I., Khurshid, M., Muzammil, S., Rasool, M. H., Nisar, M. A., Alvi, R. F., Aslam, M. A., Qamar, M. U., Salamat, M. K. F., & Baloch, Z. (2018). Antibiotic resistance: a rundown of a global crisis. Infection and Drug Resistance, 11, 1645–1658.
Jasovský, D., Littman, J., Zorzet, A., & Cars, O. (2016). Antimicrobial resistance—a threat to the world’s sustainable development. Upsala Journal of Medical Sciences, 121(3), 159–164.
OECD (2018). Stemming the superbug tide: just a few dollars more. Paris: OECD Publishing. https://doi.org/10.1787/9789264307599-en.
Conlon, B. P., Nakayasu, E. S., Fleck, L. E., LaFleur, M. D., Isabella, V. M., Coleman, K., Leonard, S. N., Smith, R. D., Adkins, J. N., & Lewis, K. (2013). Killing persister cells and eradicating a biofilm infection by activating the ClpP protease. Nature, 21, 365–370. https://doi.org/10.1038/nature12790.
Olsen, I.(2015). Biofilm-specific antibiotic tolerance and resistance. European Journal of Clinical Microbiology & Infectious Diseases, 34, 877–886.
Martinez, J. L., & Rojo, F. (2011). Metabolic regulation of antibiotic resistance. FEMS Microbiology Reviews, 35, 768–789.
Yan, J., & Bassler, B. L. (2019). Surviving as a community: antibiotic tolerance and persistence in bacterial biofilms. Cell Host & Microbe, 26, 15–21.
Bowler P., Murphy C., Wolcott R. (2020) Biofilm exacerbates antibiotic resistance: Is this a current oversight in antimicrobial stewardship? Antimicrobial Resistance & Infection Control, 9, 162. https://doi.org/10.1186/s13756-020-00830-6
Costerton, J. W., Montanaro, L., & Arciola, C. R. (2005). Biofilm in implant infections: its production and regulation. The International Journal of Artificial Organs, 28, 1062–1068.
Hoiby, N., Ciofu, O., Johansen, H. K., Song, Z. J., Moser, C., Jensen, P. Ø., Molin, S., Givskov, M., Tolker-Nielsen, T., & Bjarnsholt, T. (2011). The clinical impact of bacterial biofilms. International Journal of Oral Science, 3, 55.
Sharma, D., Misba, L. & Khan, A.U. (2019) Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrobial Resistance & Infection Control, 8, 76. https://doi.org/10.1186/s13756-019-0533-3
Whitchurch, C. B., Tolker-Nielsen, T., Ragas, P. C., & Mattick, J. S. (2002). Extracellular DNA required for bacterial biofilm formation. Science, 295, 1487.
Wingender, J., Strathmann, M., Rode, A., Leis, A., & Flemming, H. C. (2001). Isolation and biochemical characterization of extracellular polymeric substances from Pseudomonas aeruginosa. Methods in Enzymology, 336, 302–314.
Donlan, R. M. (2002). Biofilms: microbial life on surfaces. Emerging Infectious Diseases, 8(9), 881–890.
Kostakioti, M., Hadjifrangiskou, M., & Hultgren, S. J. (2013). Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the post antibiotic era. Cold Spring Harbor Perspectives in Medicine, 3(4), a010306.
Borlee, B. R., Goldman, A. D., Murakami, K., Samudrala, R., Wozniak, D. J., & Parsek, M. R. (2010). Pseudomonas aeruginosa uses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrix. Molecular Microbiology, 75(4), 827–842.
Ahmad, I., Nygren, E., Khalid, F., Myint, S. L., & Uhlin, B. E. (2020). A Cyclic-di-GMP signalling network regulates biofilm formation and surface-associated motility of Acinetobacter baumannii 17978. Scientific Reports, 10(1), 1991.
Van Houdt, R., Givskov, M., & Michiels, C. W. (2007). Quorum sensing in Serratia. FEMS Microbiology Reviews, 31(4), 407–424.
Del Pozo, J. L. (2018). Biofilm-related disease. Expert Review of Anti-Infective Therapy, 16(1), 51–65.
Lino, C. A., Harper, J. C., Carney, J. P., & Timlin, J. A. (2018). Delivering CRISPR: a review of the challenges and approaches. Drug Delivery, 25(1), 1234–1257.
Nidhi, S., Anand, U., Oleksak, P., Tripathi, P., Lal, J. A., Thomas, G., Kuca, K., & Tripathi, V. (2021). Novel CRISPR-Cas Systems: An updated review of the current achievements, applications, and future research perspectives. International Journal of Molecular Sciences, 22(7), 3327 https://doi.org/10.3390/ijms22073327.
Ishino, Y., Krupovic, M., & Forterre, P. (2018). History of CRISPR-Cas from an encounter with a mysterious repeated sequence to genome editing technology. Journal of Bacteriology, 200(7), e00580–17. https://doi.org/10.1128/JB.00580-17.
Han, W., & She, Q. (2017). CRISPR History: Discovery, characterization, and prosperity. Progress in Molecular Biology and Translational Science, 152, 1–21.
Bolotin, A., Quinquis, B., Sorokin, A., & Ehrlich, S. D. (2005). Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology, 151(8), 2551–2561.
Jansen, R., Embden, J. D., Gaastra, W., & Schouls, L. M. (2002). Identification of genes that are associated with DNA repeats in prokaryotes. Molecular Microbiology, 43(6), 1565–1575.
Barrangou, R., Fremaux, C., Deveau, H., Richards, M., Boyaval, P., Moineau, S., Romero, D. A., & Horvath, P. (2007). CRISPR provides acquired resistance against viruses in prokaryotes. Science, 315(5819), 1709–1712.
Horvath, P., & Barrangou, R. (2010). CRISPR/Cas, the immune system of bacteria and archaea. Science, 327(5962), 167–170.
Lander, E. S. (2016). The heroes of CRISPR. Cell, 164(1-2), 18–28.
Marraffini, L. A., & Sontheimer, E. J. (2008). CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science, 322(5909), 1843–1845.
Hale, C. R., Zhao, P., Olson, S., Duff, M. O., Graveley, B. R., Wells, L., Terns, R. M., & Terns, M. P. (2009). RNA-guided RNA cleavage by a CRISPR RNA-Cas protein complex. Cell, 139(5), 945–956.
Garneau, J. E., Dupuis, M. È., Villion, M., Romero, D. A., Barrangou, R., Boyaval, P., Fremaux, C., Horvath, P., Magadán, A. H., & Moineau, S. (2010). The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature, 468(7320), 67–71.
Makarova, K. S., Haft, D. H., Barrangou, R., Brouns, S. J., Charpentier, E., Horvath, P., Moineau, S., Mojica, F. J., Wolf, Y. I., Yakunin, A. F., van der Oost, J., & Koonin, E. V. (2011). Evolution and classification of the CRISPR-Cas systems. Nature Reviews Microbiology, 9(6), 467–477. https://doi.org/10.1038/nrmicro2577.
Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J. A., & Charpentier, E. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science, 337(6096), 816–821.
Deltcheva, E., Chylinski, K., Sharma, C. M., Gonzales, K., Chao, Y., Pirzada, Z. A., Eckert, M. R., Vogel, J., & Charpentier, E. (2011). CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature, 471(7340), 602–607.
Abbott, A. (2016). The Quiet Revolutionary: How the co-discovery of CRISPR explosively changed Emmanuelle Charpentier’s life. Nature, 532(7600), 432–434.
Sapranauskas, R., Gasiunas, G., Fremaux, C., Barrangou, R., Horvath, P., & Siksnys, V. (2011). The Streptococcus thermophilus CRISPR/Cas system provides immunity in Escherichia coli. Nucleic Acids Research, 39(21), 9275–9282.
Cong, L., Ran, F. A., Cox, D., Lin, S., Barretto, R., Habib, N., Hsu, P. D., Wu, X., Jiang, W., Marraffini, L. A., & Zhang, F. (2013). Multiplex genome engineering using CRISPR/Cas systems. Science, 339(6121), 819–823.
Mali, P., Yang, L., Esvelt, K. M., Aach, J., Guell, M., DiCarlo, J. E., Norville, J. E., & Church, G. M. (2013). RNA-guided human genome engineering via Cas9. Science, 339(6121), 823–826.
Golkar, Z. (2020). CRISPR: a journey of gene-editing based medicine. Genes Genomics, 42(12), 1369–1380.
Makarova, K. S., Wolf, Y. I., Iranzo, J., Shmakov, S. A., Alkhnbashi, O. S., Brouns, S. J. J., Charpentier, E., Cheng, D., Haft, D. H., Horvath, P., Moineau, S., Mojica, F. J. M., Scott, D., Shah, S. A., Siksnys, V., Terns, M. P., Venclovas, Č., White, M. F., Yakunin, A. F., Yan, W., Zhang, F., Garrett, R. A., Backofen, R., van der Oost, J., Barrangou, R. & Koonin, E. V. (2020). Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nature Reviews Microbiology, 18(2), 67–83.
Jore, M. M., Lundgren, M., van Duijn, E., Bultema, J. B., Westra, E. R., Waghmare, S. P., Wiedenheft, B., Pul, U., Wurm, R., Wagner, R., Beijer, M. R., Barendregt, A., Zhou, K., Snijders, A. P., Dickman, M. J., Doudna, J. A., Boekema, E. J., Heck, A. J., van der Oost, J., & Brouns, S. J. (2011). Structural basis for CRISPR RNA-guided DNA recognition by Cascade. Nature Structural & Molecular Biology, 18(5), 529–536.
Newsom, S., Parameshwaran, H. P., Martin, L., & Rajan, R. (2020). The CRISPR-Cas mechanism for adaptive immunity and alternate bacterial functions fuels diverse biotechnologies. Frontiers in Cellular and Infection Microbiology, 10, 619763.
Pinilla-Redondo, R., Mayo-Munoz, D., Russel, J., Garrett, R. A., Randau, L., Sorensen, S. J., & Shah, S. A. (2020). Type IV CRISPR-Cas systems are highly diverse and involved in competition between plasmids. Nucleic Acids Research, 48(4), 2000–2012.
Harrington, L. B., Ma, E., Chen, J. S., Witte, I. P., Gertz, D., Paez-Espino, D., Al-Shayeb, B., Kyrpides, N. C., Burstein, D., Banfield, J. F., & Doudna, J. A. (2020). A scoutRNA is required for some type V CRISPR-Cas systems. Molecular Cell, 79(3), 416–24 e5.
O’Connell, M. R. (2019). Molecular mechanisms of RNA targeting by Cas13-containing Type VI CRISPR-Cas systems. Journal of Molecular Biology, 431(1), 66–87.
Shabbir, M. A., Hao, H., Shabbir, M. Z., Hussain, H. I., Iqbal, Z., Ahmed, S., Sattar, A., Iqbal, M., Li, J., & Yuan, Z. (2016). Survival and evolution of CRISPR-Cas System in prokaryotes and its applications. Frontiers in Immunology, 7, 375.
Asmamaw, M., & Zawdie, B. (2021). Mechanism and applications of CRISPR/Cas-9-mediated genome editing. Biologics, 15, 353–361.
Bikard, D., Euler, C. W., Jiang, W., Nussenzweig, P. M., Goldberg, G. W., Duportet, X., Fischetti, V. A., & Marraffini, L. A. (2014). Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nature Biotechnology, 32, 1146–1150.
Kim, J. S., Cho, D. H., Park, M., Chung, W. J., Shin, D., Ko, K. S., & Kweon, D. H. (2016). CRISPR/Cas9-mediated re-sensitization of antibiotic-resistant Escherichia coli harboring extended-spectrum beta-lactamases. Journal of Microbiology and Biotechnology, 26, 394–401.
Price, V. J., McBride, S. W., Hullahalli, K., Chatterjee, A., Duerkop, B. A., & Palmer, K. L. (2019). Enterococcus faecalis CRISPR-Cas is a robust barrier to conjugative antibiotic resistance dissemination in the murine intestine. mSphere, 4, e00464–19.
Rodrigues, M., McBride, S. W., Hullahalli, K., Palmer, K. L., & Duerkop, B. A. (2019). Conjugative delivery of CRISPR-Cas9 for the selective depletion of antibiotic-resistant Enterococci. Antimicrobial Agents and Chemotherapy, 63, e01454–19.
Wan, F., Draz, M. S., Gu, M., Yu, W., Ruan, Z., & Luo, Q. (2021). Novel strategy to combat antibiotic resistance: A sight into the combination of CRISPR/Cas9 and nanoparticles. Pharmaceutics, 13(3), 352 https://doi.org/10.3390/pharmaceutics13030352.
Gomaa, A. A., Klumpe, H. E., Luo, M. L., Selle, K., Barrangou, R., & Beisel, C. L. (2014). Programmable removal of bacterial strains by use of genome-targeting CRISPR-Cas systems. MBio, 5(1), 10–1128.
Kiga, K., Tan, X. E., Ibarra-Chávez, R., Watanabe, S., Aiba, Y., Sato’o, Y., Li, F. Y., Sasahara, T., Cui, B., Kawauchi, M., Boonsiri, T., Thitiananpakorn, K., Taki, Y., Azam, A. H., Suzuki, M., Penadés, J. R., & Cui, L. (2020). Development of CRISPR-Cas13a-based antimicrobials capable of sequence-specific killing of target bacteria. Nature Communication, 11(1), 2934.
Kang, Y. K., Kwon, K., Ryu, J. S., Lee, H. N., Park, C., & Chung, H. J. (2017). Nonviral genome editing based on a polymer-derivatized CRISPR nano complex for targeting bacterial pathogens and antibiotic resistance. Bioconjugate Chemistry, 28(4), 957–967.
Yosef, I., Manor, M., Kiro, R., & Qimron, U. (2015). Temperate and lytic bacteriophages programmed to sensitize and kill antibiotic-resistant bacteria. Proceedings of the National Academy of Sciences of the United States of America, 112(23), 7267–7272.
Citorik, R. J., Mimee, M., & Lu, T. K. (2014). Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nature Biotechnology, 32(11), 1141–1145.
Tagliaferri, T. L., Guimarães, N. R., Pereira, M. D. P. M., Horz, H. P., & Mendes, T. A. D. O. (2020). Exploring the potential of CRISPR-Cas9 under challenging conditions: facing high-copy plasmids and counteracting beta-lactam resistance in clinical strains of Enterobacteriaceae. Frontiers in Microbiology, 11, 511912.
Rodrigues, M., McBride, S. W., Hullahalli, K., Palmer, K. L., & Duerkop, B. A. (2019). Conjugative delivery of CRISPR-Cas9 for the selective depletion of antibiotic-resistant enterococci. Antimicrobial Agents and Chemotherapy, 63(11), 10–1128.
Hao, M., He, Y., Zhang, H., Liao, X. P., Liu, Y. H., Sun, J., Du, H., Kreiswirth, B. N., & Chen, L. (2020). CRISPR-Cas9-mediated carbapenemase gene and plasmid curing in carbapenem-resistant Enterobacteriaceae. Antimicrobial Agents and Chemotherapy, 64(9), 10–1128.
Liu, H., Li, H., Liang, Y., Du, X., Yang, C., Yang, L., Xie, J., Zhao, R., Tong, Y., Qiu, S., & Song, H. (2020). Phage-delivered sensitisation with subsequent antibiotic treatment reveals sustained effect against antimicrobial resistant bacteria. Theranostics, 10(14), 6310.
Singla, S., Harjai, K., Katare, O. P., & Chhibber, S. (2016). Encapsulation of bacteriophage in liposome accentuates its entry in to macrophage and shields it from neutralizing antibodies. PLoS ONE, 11(4), e0153777.
Cobb, L. H., Park, J., Swanson, E. A., Beard, M. C., McCabe, E. M., Rourke, A. S., Seo, K. S., Olivier, A. K., & Priddy, L. B. (2019). CRISPR-Cas9 modified bacteriophage for treatment of Staphylococcus aureus induced osteomyelitis and soft tissue infection. PLoS ONE, 14(11), e0220421.
Zuberi, A., Ahmad, N., & Khan, A. U. (2017). CRISPRi induced suppression of fimbriae gene (fimH) of a uropathogenic Escherichia coli: An approach to inhibit microbial biofilms. Frontiers in Immunology, 8, 1552.
Kang, S., Kim, J., Hur, J. K., & Lee, S. S. (2017). CRISPR-based genome editing of clinically important Escherichia coli SE15 isolated from indwelling urinary catheters of patients. Journal of Medical Microbiology, 66(1), 18–25. https://doi.org/10.1099/jmm.0.000406.
Gholizadeh, P., Kose, S., Dao, S., Ganbarov, K., Tanomand, A., Dal, T., Aghazadeh, M., Ghotaslou, R., Ahangarzadeh Rezaee, M., Yousefi, B., & Samadi Kafil, H. (2020). How CRISPR-Cas system could be used to combat antimicrobial resistance. Infection and Drug Resistance, 13, 1111–1121.
Hegde, S., Nilyanimit, P., Kozlova, E., Anderson, E. R., Narra, H. P., Sahni, S. K., Heinz, E., & Hughes, G. L. (2019). CRISPR/Cas9-mediated gene deletion of the ompA gene in symbiotic Cedecea neteri impairs biofilm formation and reduces gut colonization of Aedes aegypti mosquitoes. PLOS Neglected Tropical Diseases, 13(12), e0007883.
Gholizadeh, P., Aghazadeh, M., Asgharzadeh, M., & Kafil, H. S. (2017). Suppressing the CRISPR/Cas adaptive immune system in bacterial infections. European Journal of Clinical Microbiology and Infectious Diseases, 36, 2043–2051. https://doi.org/10.1007/s10096-017-3036-2.
Yao, R., Liu, D., Jia, X., Zheng, Y., Liu, W., & Xiao, Y. (2018). CRISPR-Cas9/Cas12a biotechnology and application in bacteria. Synthetic and Systems Biotechnology, 3, 135–149.
Jiang, W., Bikard, D., Cox, D., Zhang, F., & Marraffini, L. A. (2013). RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nature Biotechnology, 31, 233–239. https://doi.org/10.1038/nbt.2508.
Goren, M., Yosef, I., & Qimron, U. (2017). Sensitizing pathogens to antibiotics using the CRISPR-Cas system. Drug Resistance Updates, 30, 1–6. https://doi.org/10.1016/j.drup.2016.11.001.
Touchon, M., Charpentier, S., Pognard, D., Picard, B., Arlet, G., Rocha, E. P., Denamur, E., & Branger, C. (2012). Antibiotic resistance plasmids spread among natural isolates of Escherichia coli in spite of CRISPR elements. Microbiology, 158, 2997–3004. https://doi.org/10.1099/mic.0.060814-0.
Hale, C. R., Majumdar, S., Elmore, J., Pfister, N., Compton, M., Olson, S., Resch, A. M., Glover, 3rd, C. V., Graveley, B. R., Terns, R. M., & Terns, M. P. (2012). Essential features and rational design of CRISPR RNAs that function with the Cas RAMP module complex to cleave RNAs. Molecular Cell, 45, 292–302. https://doi.org/10.1016/j.molcel.2011.10.023.
Zuberi, A., Misba, L. & & Khan, A. U. (2017). CRISPR interference (CRISPRi) inhibition of luxS gene expression in E. coli: An approach to inhibit biofilm. Frontiers in Cellular and Infection Microbiology, 7, 214 https://doi.org/10.3389/fcimb.2017.00214.
Gong, T., Tang, B., Zhou, X., Zeng, J., Lu, M., Guo, X., Peng, X., Lei, L., Gong, B., & Li, Y. (2018). Genome editing in Streptococcus mutans through self-targeting CRISPR arrays. Molecular Oral Microbiology, 33, 440–449. https://doi.org/10.1111/omi.12247.
Garrido, V., Pinero-Lambea, C., Rodriguez-Arce, I., Paetzold, B., Ferrar, T., Weber, M., Garcia-Ramallo, E., Gallo, C., Collantes, M., Penuelas, I., Serrano, L., Grilló, M. J., & Lluch-Senar, M. (2021). Engineering a genome-reduced bacterium to eliminate Staphylococcus aureus biofilms in vivo. Molecular Systems Biology, 17, e10145 https://doi.org/10.15252/msb.202010145.
Sharma, S., Mohler, J., Mahajan, S. D., Schwartz, S. A., Bruggemann, L., & Aalinkeel, R. (2023). Microbial Biofilm: A review on formation, infection, antibiotic resistance, control measures, and innovative treatment. Microorganisms, 19, 1614 https://doi.org/10.3390/microorganisms11061614.
Gupta, S., Kumar, P., Rathi, B., Verma, V., Dhanda, R. S., Devi, P., & Yadav, M. (2021). Targeting of uropathogenic Escherichia coli papG gene using CRISPR-dot nanocomplex reduced virulence of UPEC. Scientific Reports, 11(1), 17801.
Acknowledgements
The authors thank UM DAE Center for Excellence in Basic Sciences, Mumbai, India, for supporting this work.
Author information
Authors and Affiliations
Contributions
P.P.—Literature survey, summarized and wrote the draft of the review. V.L.S.—Conceptualization, design, discussion, literature survey, editing, and finalizing the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pandey, P., Vavilala, S.L. From Gene Editing to Biofilm Busting: CRISPR-CAS9 Against Antibiotic Resistance—A Review. Cell Biochem Biophys (2024). https://doi.org/10.1007/s12013-024-01276-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s12013-024-01276-y