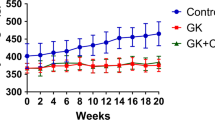
Abstract
This study aims to characterize the bone-protecting effects of Alpha-lipoic acid (ALA), a potent antioxidant, against the detrimental effects of the coexistence of type 2 diabetes mellitus (T2DM) and postmenopausal osteoporosis (POP) and identify the possible mechanisms with particular reference to its modulation of YAP/Glut4 pathway. The T2DM and POP coexisting model was induced in mice by high fat diet (HFD) + Streptozocin (STZ) + ovariectomy (OVX). The mice in the treatment groups were given ALA for 10 weeks. In the in vitro study, MC3T3-E1 cells were induced with 500 μM methylglyoxal for 24 h with or without pretreatment with ALA for 24 h. The oxidative and antioxidative biomarkers, bone microarchitecture, histo-morphology, and related protein expression of apoptosis, osteogenic differentiation and the YAP/Glut4 pathway were detected. The results showed ALA could improve glucose tolerance, inhibit oxidative stress and apoptosis and alleviate bone loss. Further study by siRNA technology revealed that the YAP/Glut4 pathway was implicated in the pathogenesis of bone loss due to the coexistence of T2DM and POP. Taken together, the present study has demonstrated for the first time that ALA exerts potent protective effects against bone loss in T2DM and POP coexisting conditions by modulating the YAP/Glut4 pathway.












Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding authors upon request.
Abbreviations
- ALA:
-
alpha-lipoic acid
- T2DM:
-
type 2 diabetes mellitus
- POP:
-
postmenopausal osteoporosis
- HFD:
-
high fat diet
- STZ:
-
streptozocin
- OVX:
-
ovariectomy
- OP:
-
osteoporosis
- ER:
-
estrogen receptor
- BMD:
-
bone mineral density
- MG:
-
Methylglyoxal
- FBS:
-
fetal bovine serum
- FBG:
-
fasting blood glucose
- PBG:
-
postprandial blood glucose
- BV:
-
bone volume
- BS:
-
bone surface
- ROI:
-
region of interest
- GP:
-
growth plate
- HE:
-
hematoxylin and eosin
- LDH:
-
lactate dehydrogenase
- SOD:
-
superoxide dismutase
- MDA:
-
malonaldehyde
- GSH:
-
glutathione
- DCFH-DA:
-
2,7-dichlorofluorescein diacetate
- PVDF:
-
polyvinyl difluoride
- EMD:
-
membranes
- TBS:
-
tris-buffered saline
- LSD:
-
least significant difference
- OGTT:
-
oral glucose tolerance test
- GP:
-
growth plate
References
Xiao, L., et al. (2015). Changes of serum osteocalcin, calcium, and potassium in a rat model of type 2 diabetes. Cell Biochem Biophys, 71(1), 437–440.
Zhao, R. (2012). Immune regulation of osteoclast function in postmenopausal osteoporosis: a critical interdisciplinary perspective. International Journal of Medical Sciences, 9(9), 825–832.
Díaz, A., et al. (2019). Sex differences in age-associated type 2 diabetes in rats — Role of estrogens and oxidative stress. Oxidative Medicine and Cellular Longevity, 2019, 6734836.
Mastrandrea, L. D., et al. (2008). Young women with type 1 diabetes have lower bone mineral density that persists over time. Diabetes Care, 31(9), 1729–1735.
Zhou, R. K., Wang, Z. H., & Chao, M. (2014). Hispidulin exerts anti-osteoporotic activity in ovariectomized mice via activating AMPK signaling pathway. Cell Biochemistry and Biophysics, 69(2), 311–317.
Napoli, N., et al. (2017). Mechanisms of diabetes mellitus-induced bone fragility. Nature Reviews. Endocrinology, 13(4), 208–219.
Li, Z., et al. (2016). Glucose transporter-4 facilitates insulin-stimulated glucose uptake in osteoblasts. Endocrinology, 157(11), 4094–4103.
Packer, L., Witt, E. H., & Tritschler, H. J. (1995). Alpha-Lipoic acid as a biological Antioxidant. Free Radical Biology and Medicine, 19(2), 227–250.
Salehi, B., et al. (2019). Insights on the Use of α-Lipoic Acid for Therapeutic Purposes. Biomolecules, 9(8), 356.
Smith, A. R., et al. (2004). Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Current Medicinal Chemistry, 11(9), 1135–1146.
Wollin, S. D., & Jones, P. J. (2003). Alpha-lipoic acid and cardiovascular disease. Jornal of Nutrition, 133(11), 3327–3330.
Gomes, M. B., & Negrato, C. A. (2014). Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetology & Metabolic Syndrome, 6(1), 80.
Fu, C., et al. (2015). Alpha-lipoic acid promotes osteoblastic formation in H2O2-treated MC3T3-E1 cells and prevents bone loss in ovariectomized rats. Journal of Cellular Physiology, 230(9), 2184–2201.
Raehtz, S., et al. (2017). Estrogen Deficiency Exacerbates Type 1 Diabetes–Induced Bone TNF-α Expression and Osteoporosis in Female Mice. Endocrinology, 158(7), 2086–2101.
Kanazawa, I., & Sugimoto, T. (2018). Diabetes mellitus-induced bone fragility. Internal Medicine, 57(19), 2773–2785.
Lee, Y. S., et al. (2018). Effect of a bisphosphonate and selective estrogen receptor modulator on bone remodeling in streptozotocin-induced diabetes and ovariectomized rat model. Spine Journal, 18(10), 1877–1887.
Li, Y., et al. (2018). Preventative effects of resveratrol and estradiol on streptozotocin-induced diabetes in ovariectomized mice and the related mechanisms. PLoS One, 13(10), e0204499.
Lu, S., et al. (2017). The osteogenesis-promoting effects of alpha-lipoic acid against glucocorticoid-induced osteoporosis through the NOX4, NF-kappaB, JNK and PI3K/AKT pathways. Scientific Reports, 7(1), 3331.
Mukaiyama, K., et al. (2015). Elevation of serum alkaline phosphatase (ALP) level in postmenopausal women is caused by high bone turnover. Aging Clinical and Experimental Research, 27(4), 413–418.
Oka, Y., et al. (2012). Tea polyphenols inhibit rat osteoclast formation and differentiation. Journal of Pharmacological Sciences, 118(1), 55–64.
Xie, H., et al. (2018). Possible therapeutic potential of berberine in the treatment of STZ plus HFD-induced diabeticosteoporosis. Biomedicien. Pharmacotherapy, 108, 280–287.
Tüzün, Ş., et al. (2013). Impact of the training on the compliance and persistence of weekly bisphosphonate treatment in postmenopausal osteoporosis: a randomized controlled study. International Journal of Medical Sciences, 10(13), 1880–1887.
Wang, J., et al. (2016). Chondroprotective effects of alpha-lipoic acid in a rat model of osteoarthritis. Free Radical Research, 50(7), 767–780.
Desai, K., & Wu, L. (2007). Methylglyoxal and advanced glycation endproducts: new therapeutic horizons? Recent Patents on Cardiovascular Drug Discovery, 2(2), 88–89.
Vander Jagt, D. L. (2008). Methylglyoxal, diabetes mellitus and diabetic complications. Drug Metabolism and Drug Interactions, 23(1-2), 93–124.
Dhar, A., et al. (2011). Chronic methylglyoxal infusion by minipump causes pancreatic beta-cell dysfunction and induces type 2 diabetes in Sprague-Dawley rats. Diabetes, 60(3), 899–908.
Chu, P., et al. (2017). Phosphocreatine protects endothelial cells from Methylglyoxal induced oxidative stress and apoptosis via the regulation of PI3K/Akt/eNOS and NF-κB pathway. Vascular Pharmacology, 91, 26–35.
Lian, I., et al. (2010). The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Development, 24(11), 1106–1118.
He, C., et al. (2015). The Hippo/YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression. EMBO Molecular Medicine, 7(11), 1426–1449.
Zhao, B., Lei, Q., & Guan, K. (2008). The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Current Opinion in Cell Biology, 20(6), 638–646.
Moroishi, T., et al. (2016). The Hippo Pathway Kinases LATS1/2 Suppress Cancer Immunity. Cell, 167(6), 1525–1539.
Collak, F. K., et al. (2017). Increased expression of YAP1 in prostate cancer correlates with extraprostatic extension. Cancer Biology & Medicine, 14(4), 405–413.
Ma, R., et al. (2019). Activated YAP causes renal damage of type 2 diabetic nephropathy. European Review for Medical and Pharmacological Sciences, 23(2), 755–763.
Larance, M., Ramm, G., & James, D. E. (2008). The GLUT4 code. Molecular Endocrinology, 22(2), 226–233.
Lee, J. O., et al. (2012). Metformin regulates glucose transporter 4 (GLUT4) translocation through AMP-activated protein kinase (AMPK)-mediated Cbl/CAP signaling in 3T3-L1 preadipocyte cells. Journal of Biological Chemistry, 287(53), 44121–44129.
Castaneda, F., Layne, J. E., & Castaneda, C. (2006). Skeletal muscle sodium glucose co-transporters in older adults with type 2 diabetes undergoing resistance training. International Journal of Medical Sciences, 3(3), 84–91.
Author contributions
All authors contributed to the study conception and design. H.S. and M.L. conceived and designed this study. L.X. and C.Z. performed the major experiments and data analysis and drafted the manuscript. C.W., J.B., G.H., Y.C. and G.X. provided technological support. All the authors have read and approved this manuscript.
Funding
The work was supported in part by grants from Important Science Fund of Science and Technology Bureau of Liaoning Province (2020JH2/10300056) and Fund of Department of Education of Liaoning Provincial (No. LZ2019016).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, L., Zhang, C., Bao, J. et al. Alpha-lipoic Acid Prevents Bone Loss in Type 2 Diabetes and Postmenopausal Osteoporosis Coexisting Conditions by Modulating the YAP/Glut4 Pathway. Cell Biochem Biophys (2024). https://doi.org/10.1007/s12013-024-01216-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12013-024-01216-w