Abstract
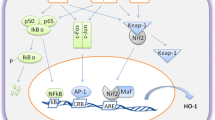
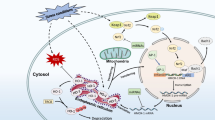
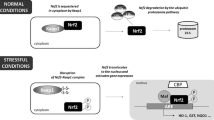
HO-1 is the inducible form of the enzyme heme-oxygenase. HO-1 catalyzes heme breakdown, reducing the levels of this important oxidant molecule and generating antioxidant, anti-inflammatory, and anti-apoptotic byproducts. Thus, HO-1 has been described as an important stress response mechanism during both physiologic and pathological processes. Interestingly, some findings are demonstrating that uncontrolled levels of HO-1 byproducts can be associated with cell death and tissue destruction as well. Furthermore, HO-1 can be located in the nucleus, influencing gene transcription, cellular proliferation, and DNA repair. Here, we will discuss several studies that approach HO-1 effects as a protective or detrimental mechanism in different pathological conditions. In this sense, as the major organs of vertebrates will deal specifically with distinct types of stresses, we discuss the HO-1 role in each of them, exposing the contradictions associated with HO-1 expression after different insults and circumstances.

Similar content being viewed by others
Change history
06 December 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12013-021-01053-1
References
Tenhunen, R., Marver, H. S., & Schmid, R. (1968). The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proceedings of the National Academy of Sciences of the United States of America., 61(2 Oct), 748–55. https://doi.org/10.1073/pnas.61.2.748. PMID: 4386763; PMCID: PMC225223.
Kohchi, T., Mukougawa, K., Frankenberg, N., Masuda, M., Yokota, A., & Lagarias, J. C. (2001). The Arabidopsis HY2 gene encodes phytochromobilin synthase, a ferredoxin-dependent biliverdin reductase. The Plant Cell, 13(2), 425–436. https://doi.org/10.1105/tpc.13.2.425.
Maines, M. D. (1997). The heme oxygenase system: a regulator of second messenger gases. Annual Review of Pharmacology and Toxicology, 37, 517–54. https://doi.org/10.1146/annurev.pharmtox.37.1.517. PMID: 9131263.
Martínez-Casales, M., Hernanz, R., & Alonso, M. J. (2021). Vascular and macrophage heme oxygenase-1 in hypertension: a mini-review. Frontiers in Physiology, 26(12 Feb), 643435. https://doi.org/10.3389/fphys.2021.643435. PMID: 33716792; PMCID: PMC7952647.
Ishii, T., Itoh, K., Takahashi, S., Sato, H., Yanagawa, T., Katoh, Y., Bannai, S., & Yamamoto, M. (2000). Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. Journal of Biological Chemistry, 275(21 May), 16023–9. https://doi.org/10.1074/jbc.275.21.16023. PMID: 10821856.
Biswas, C., Shah, N., & Muthu, M., et al. (2014). Nuclear heme oxygenase-1 (HO-1) modulates subcellular distribution and activation of Nrf2, impacting metabolic and anti-oxidant defenses. Journal of Biological Chemistry, 289(39), 26882–26894. https://doi.org/10.1074/jbc.M114.567685.
Lin, Q., Weis, S., Yang, G., Weng, Y. H., Helston, R., Rish, K., Smith, A., Bordner, J., Polte, T., Gaunitz, F. & Dennery, P. A. (2007). Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. Journal of Biological Chemistry, 282(28 Jul), 20621–33. https://doi.org/10.1074/jbc.M607954200. Epub 2007 Apr 12. PMID: 17430897.
Bauer, M., & Bauer, I. (2002). Heme oxygenase-1: redox regulation and role in the hepatic response to oxidative stress. Antioxidants & Redox Signaling, 4(5 Oct), 749–58. https://doi.org/10.1089/152308602760598891. PMID: 12470502.
Möbius, K., Arias-Cartin, R., & Breckau, D., et al. (2010). Heme biosynthesis is coupled to electron transport chains for energy generation. Proceedings of the National Academy of Sciences of the United States of America, 107(23), 10436–10441. https://doi.org/10.1073/pnas.1000956107.
Zuckerbraun, B. S., Chin, B. Y., Bilban, M., d’Avila, J. C., Rao, J., Billiar, T. R. & Otterbein, L. E. (2007). Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. The FASEB Journal, 21(4 Apr), 1099–106. https://doi.org/10.1096/fj.06-6644com. Epub 2007 Jan 30. PMID: 17264172.
Vanella, L., Barbagallo, I., Tibullo, D., Forte, S., Zappalà, A., Li, & Volti, G. (2016). The non-canonical functions of the heme oxygenases. Oncotarget, 7(42 Oct), 69075–69086. https://doi.org/10.18632/oncotarget.11923. PMID: 27626166; PMCID: PMC5356613.
Carr, J. F., Garcia, D., Scaffa, A., Peterson, A. L., Ghio, A. J., & Dennery, P. A. (2020). Heme oxygenase-1 supports mitochondrial energy production and electron transport chain activity in cultured lung epithelial cells. International Journal of Molecular Sciences, 21(18 Sep), 6941. https://doi.org/10.3390/ijms21186941. PMID: 32971746; PMCID: PMC7554745.
Dunn, L. L., Midwinter, R. G., Ni, J., Hamid, H. A., Parish, C. R., & Stocker, R. (2014). New insights into intracellular locations and functions of heme oxygenase-1. Antioxidants & Redox Signaling, 20(11), 1723–1742. https://doi.org/10.1089/ars.2013.5675.
Ryter, S. W. (2019). Heme oxygenase-1/carbon monoxide as modulators of autophagy and inflammation. Archieves of Biochemistry and Biophysics, 678(Dec), 108186. https://doi.org/10.1016/j.abb.2019.108186. Epub 2019 Nov 5. PMID: 31704095.
Silva, R. C. M. C., Travassos, L. H., Paiva, C. N., & Bozza, M. T. (2020). Heme oxygenase-1 in protozoan infections: a tale of resistance and disease tolerance. PLoS Pathogens, 16(7 Jul), e1008599. https://doi.org/10.1371/journal.ppat.1008599. PMID: 32692767; PMCID: PMC7373268.
Costa, D. L., Amaral, E. P., Andrade, B. B., & Sher, A. (2020). Modulation of inflammation and immune responses by heme oxygenase-1: implications for infection with intracellular Pathogens. Antioxidants (Basel), 9(12 Nov), 1205 https://doi.org/10.3390/antiox9121205. PMID: 33266044; PMCID: PMC7761188.
Alam, J., & Cook, J. L. (2003). Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Current Pharmaceutical Design, 9(30), 2499–511. https://doi.org/10.1165/rcmb.2006-0340TR. PMID: 14529549.
Sun, J., Hoshino, H., Takaku, K., Nakajima, O., Muto, A., Suzuki, H., Tashiro, S., Takahashi, S., Shibahara, S., Alam, J., Taketo, M. M., Yamamoto, M., & Igarashi, K. (2002). Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. The EMBO Journal, 21(19 Oct), 5216–24. https://doi.org/10.1093/emboj/cdf516. PMID: 12356737; PMCID: PMC129038.
Dhakshinamoorthy, S., Jain, A. K., Bloom, D. A. & Jaiswal, A. K. (2005). Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. Journal of Biological Chemistry, 280(17 Apr), 16891–900. https://doi.org/10.1074/jbc.M500166200. Epub 2005 Feb 24. PMID: 15734732.
Schreck, R., & Baeuerle, P. A. (1991). A role for oxygen radicals as second messengers. Trends in Cell Biology, 1(2–3 Aug), 39–42. https://doi.org/10.1016/0962-8924(91)90072-h. PMID: 14731549.
Lee, P. J., Jiang, B. H., Chin, B. Y., Iyer, N. V., Alam, J., Semenza, G. L., & Choi, A. M. (1997). Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. Journal of Biological Chemistry, 272(9 Feb), 5375–81. PMID: 9038135.
Rojo, A. I., Salina, M., Salazar, M., Takahashi, S., Suske, G., Calvo, V., de Sagarra, M. R., & Cuadrado, A. (2006). Regulation of heme oxygenase-1 gene expression through the phosphatidylinositol 3-kinase/PKC-zeta pathway and Sp1. Free Radical Biology & Medicine, 41(2 Jul), 247–61. https://doi.org/10.1016/j.freeradbiomed.2006.04.002. Epub 2006 Apr 21. PMID: 16814105.
Hock, T. D., Liby, K., Wright, M. M., McConnell, S., Schorpp-Kistner, M., Ryan, T. M., Agarwal, A., Jun, B. & Jun, D. (2007). Regulate human heme oxygenase-1 gene expression in renal epithelial cells. Journal of Biological Chemistry, 282(9 Mar), 6875–86. https://doi.org/10.1074/jbc.M608456200. Epub 2007 Jan 3. PMID: 17204476.
Ptasinska, A., Wang, S., Zhang, J., Wesley, R. A., & Danner, R. L. (2007). Nitric oxide activation of peroxisome proliferator-activated receptor gamma through a p38 MAPK signaling pathway. The FASEB Journal, 21(3 Mar), 950–61. https://doi.org/10.1096/fj.06-6822com. Epub 2006 Dec 28. PMID: 17197391.
Astort, F., Repetto, E. M., Rocha-Viegas, L., Mercau, M. E., Puch, S. S., Finkielstein, C. V., Pecci, A., & Cymeryng, C. B. (2016). Role of CREB on heme oxygenase-1 induction in adrenal cells: involvement of the PI3K pathway. Journal of Molecular Endocrinology, 57(2 Aug), 113–24. https://doi.org/10.1530/JME-16-0005. PMID: 27412767.
Zhou, H., Ying, X., Liu, Y., Ye, S., Yan, J., & Li, Y. (2017). Genetic polymorphism of heme oxygenase 1 promoter in the occurrence and severity of chronic obstructive pulmonary disease: a meta-analysis. Journal of Cellular and Molecular Medicine, 21(5), 894–903. https://doi.org/10.1111/jcmm.13028.
Song, F., Li, X., Zhang, M., Yao, P., Yang, N., Sun, X., Hu, F. B. & Liu, L. (2009). Association between heme oxygenase-1 gene promoter polymorphisms and type 2 diabetes in a Chinese population. American Journal of Epidemiology, 170(6 Sep), 747–56. https://doi.org/10.1093/aje/kwp196. Epub 2009 Aug 20. PMID: 19696228.
Takeda, M., Kikuchi, M., Ubalee, R., Na-Bangchang, K., Ruangweerayut, R., Shibahara, S., Imai, S., & Hirayama, K. (2005). Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to cerebral malaria in Myanmar. Japanese Journal of Infectious Diseases, 58(5 Oct), 268–71. PMID: 16249618.
Kuesap, J., & Na-Bangchang, K. (2010). Possible role of heme oxygenase-1 and prostaglandins in the pathogenesis of cerebral malaria: heme oxygenase-1 induction by prostaglandin D(2) and metabolite by a human astrocyte cell line. The Korean Journal of Parasitology, 48(1 Mar), 15–21. https://doi.org/10.3347/kjp.2010.48.1.15. Epub 2010 Mar 17PMID: 20333281; PMCID: PMC2843842.
Mendonça, V. R., Luz, N. F., Santos, N. J., Borges, V. M., Gonçalves, M. S., Andrade, B. B. & Barral-Netto, M. (2012). Association between the haptoglobin and heme oxygenase 1 genetic profiles and soluble CD163 in susceptibility to and severity of human malaria. Infection and Immunity, 80(4 Apr), 1445–54. https://doi.org/10.1128/IAI.05933-11. Epub 2012 Jan 30PMID: 22290142; PMCID: PMC3318432.
Vilander, L. M., Vaara, S. T., Donner, K. M., Lakkisto, P., Kaunisto, M. A., & Pettilä, V., FINNAKI Study Group. (2019). Heme oxygenase-1 repeat polymorphism in septic acute kidney injury. PLoS One, 14(5 May), e0217291 https://doi.org/10.1371/journal.pone.0217291. PMID: 31120979; PMCID: PMC6532969.
Vázquez-Armenta, G., González-Leal, N., Vázquez-de la Torre, J. M., Muñoz-Valle, J. F., Ramos-Márquez, M. E., Hernández-Cañaveral, I., Plascencia-Hernández, A., & Siller-López, F. (2013). Short (GT)n microsatellite repeats in the heme oxygenase-1 gene promoter are associated with antioxidant and anti-inflammatory status in Mexican pediatric patients with sepsis. The Tohoku Journal of Experimental Medicine, 231(3 Nov), 201–9. https://doi.org/10.1620/tjem.231.201. PMID: 24201221.
Du, Y., Zhang, H. & Xu, Y. et al. (2019). Association among genetic polymorphisms of GSTP1, HO-1, and SOD-3 and chronic obstructive pulmonary disease susceptibility. International Journal of Chronic Obstructive Pulmonary Disease, 14, 2081–2088. https://doi.org/10.2147/COPD.S213364. Published 2019 Sep 6.
Birkedal-Hansen, H. (1993). Role of cytokines and inflammatory mediators in tissue destruction. Journal of Periodontal Research, 28(6 Pt 2 Nov), 500–10. https://doi.org/10.1111/j.1600-0765.1993.tb02113.x. PMID: 8263720.
Yachie, A. (2021). Heme oxygenase-1 deficiency and oxidative stress: a review of 9 independent human cases and animal models. International Journal of Molecular Sciences, 22(4 Feb), 1514 https://doi.org/10.3390/ijms22041514. PMID: 33546372; PMCID: PMC7913498.
Abraham, N. G. (1991). Molecular regulation–biological role of heme in hematopoiesis. Blood Reviews, 5(1 Mar), 19–28. https://doi.org/10.1016/0268-960x(91)90004-v. PMID: 2032026.
Kovtunovych, G., Ghosh, M. C., Ollivierre, W., Weitzel, R. P., Eckhaus, M. A., Tisdale, J. F., Yachie, A., & Rouault, T. A. (2014). Wild-type macrophages reverse disease in heme oxygenase 1-deficient mice. Blood, 124(9 Aug), 1522–30. https://doi.org/10.1182/blood-2014-02-554162. Epub 2014 Jun 24PMID: 24963040; PMCID: PMC4148774.
Ferris, C. D., Jaffrey, S. R., Sawa, A., Takahashi, M., Brady, S. D., Barrow, R. K., Tysoe, S. A., Wolosker, H., Barañano, D. E., Doré, S., Poss, K. D., & Snyder, S. H. (1999). Haem oxygenase-1 prevents cell death by regulating cellular iron. Nature Cell Biology, 1(3 Jul), 152–7. https://doi.org/10.1038/11072. PMID: 10559901.
Suttner, D. M., & Dennery, P. A. (1999). Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. The FASEB Journal, 13(13 Oct), 1800–9. https://doi.org/10.1096/fasebj.13.13.1800. PMID: 10506583.
Bernardo, B. C., Weeks, K. L., Pretorius, L., & McMullen, J. R. (2010). Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacology & Therapeutics, 128(1 Oct), 191–227. https://doi.org/10.1016/j.pharmthera.2010.04.005. Epub 2010 May 12. PMID: 20438756.
Stanley, W. C., & Chandler, M. P. (2002). Energy metabolism in the normal and failing heart: potential for therapeutic interventions. Heart Failure Reviews, 7(2 Apr), 115–30. https://doi.org/10.1023/a:1015320423577. PMID: 11988636.
Grossman, W., Jones, D., & McLaurin, L. P. (1975). Wall stress and patterns of hypertrophy in the human left ventricle. Journal of Clinical Investigation, 56(1), 56–64. https://doi.org/10.1172/JCI108079.
Semenza, G. L. (2014). Hypoxia-inducible factor 1 and cardiovascular disease. Annual Review of Physiology, 76, 39–56. https://doi.org/10.1146/annurev-physiol-021113-170322.
Dawn, B., & Bolli, R. (2005). HO-1 induction by HIF-1: a new mechanism for delayed cardioprotection? American Journal of Physiology Heart and Circulatory Physiology, 289(2 Aug), H522–4. https://doi.org/10.1152/ajpheart.00274.2005. PMID: 16014614.
Datta Chaudhuri, R., Banik, A., Mandal, B., & Sarkar, S. (2021). Cardiac-specific overexpression of HIF-1α during acute myocardial infarction ameliorates cardiomyocyte apoptosis via differential regulation of hypoxia-inducible pro-apoptotic and anti-oxidative genes. Biochemical and Biophysical Research Communications, 537(Jan), 100–108. https://doi.org/10.1016/j.bbrc.2020.12.084. Epub 2020 Dec 31. PMID: 33388412.
Dunn, L. L., Kong, S. M. Y., Tumanov, S., Chen, W., Cantley, J., Ayer, A., Maghzal, G. J., Midwinter, R. G., Chan, K. H., Ng, M. K. C., & Stocker, R. (2021). Hmox1 (Heme Oxygenase-1) Protects Against Ischemia-Mediated Injury via Stabilization of HIF-1α (Hypoxia-Inducible Factor-1α). Arteriosclerosis, Thrombosis, and Vascular Biology, 41(1 Jan), 317–330. https://doi.org/10.1161/ATVBAHA.120.315393. Epub 2020 Nov 19. PMID: 33207934.
Katori, M., Buelow, R., Ke, B., Ma, J., Coito, A. J., Iyer, S., Southard, D., Busuttil, R. W., & Kupiec-Weglinski, J. W. (2002). Heme oxygenase-1 overexpression protects rat hearts from cold ischemia/reperfusion injury via an antiapoptotic pathway. Transplantation, 73(2 Jan), 287–92. https://doi.org/10.1097/00007890-200201270-00023. PMID: 11821745.
Collino, M., Pini, A., Mugelli, N., Mastroianni, R., Bani, D., Fantozzi, R., Papucci, L., Fazi, M. & Masini, E. (2013). Beneficial effect of prolonged heme oxygenase 1 activation in a rat model of chronic heart failure. Disease Models and Mechanisms, 6(4 Jul), 1012–20. https://doi.org/10.1242/dmm.011528. Epub 2013 Apr 16. PMID: 23592614; PMCID: PMC3701220.
Yet, S. F., Tian, R., Layne, M. D., Wang, Z. Y., Maemura, K., Solovyeva, M., Ith, B., Melo, L. G., Zhang, L., Ingwall, J. S., Dzau, V. J., Lee, M. E., & Perrella, M. A. (2001). Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circulation Research, 89(2 Jul), 168–73. https://doi.org/10.1161/hh1401.093314. PMID: 11463724.
Guo, Y., Stein, A. B., Wu, W. J., Tan, W., Zhu, X., Li, Q. H., Dawn, B., Motterlini, R. & Bolli, R. (2004). Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. American Journal of Physiology Heart and Circulatory Physiology, 286(5 May), H1649–53. https://doi.org/10.1152/ajpheart.00971.2003. Epub 2004 Jan 2. PMID: 14704226; PMCID: PMC3208268.
Neuzil, J., & Stocker, R. (1994). Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. Journal of Biological Chemistry, 269(24 Jun), 16712–9. PMID: 8206992.
Crowley, S. D., Gurley, S. B., Herrera, M. J., Ruiz, P., Griffiths, R., Kumar, A. P., Kim, H. S., Smithies, O., Le, T. H. & Coffman, T. M. (2006). Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proceedings of the National Academy of Sciences of the United States of America, 103(47 Nov), 17985–90. https://doi.org/10.1073/pnas.0605545103. Epub 2006 Nov 7. PMID: 17090678; PMCID: PMC1693859.
Tulis, D. A., Durante, W., Peyton, K. J., Evans, A. J., & Schafer, A. I. (2001). Heme oxygenase-1 attenuates vascular remodeling following balloon injury in rat carotid arteries. Atherosclerosis, 155(1 Mar), 113–22. https://doi.org/10.1016/s0021-9150(00)00552-9. PMID: 11223432.
Li, Y., Wang, Q., Xu, Q., Cai, S., Zhou, J., Ren, B., Sun, T., Liu, X., & Yu, H. (2014). Valsartan decreases neointimal hyperplasia in balloon-injured rat aortic arteries by upregulating HO-1 and inhibiting angiotensin II type 1 receptor. Life Sciences, 110(2 Aug), 70–6. https://doi.org/10.1016/j.lfs.2014.06.021. Epub 2014 Jul 8. PMID: 25014676.
Tongers, J., Fiedler, B., König, D., Kempf, T., Klein, G., Heineke, J., Kraft, T., Gambaryan, S., Lohmann, S. M., Drexler, H., & Wollert, K. C. (2004). Heme oxygenase-1 inhibition of MAP kinases, calcineurin/NFAT signaling, and hypertrophy in cardiac myocytes. Cardiovascular Research, 63(3 Aug), 545–52. https://doi.org/10.1016/j.cardiores.2004.04.015. PMID: 15276480.
Hull, T. D., Boddu, R., & Guo, L., et al. (2016). Heme oxygenase-1 regulates mitochondrial quality control in the heart. JCI Insight, 1(2), e85817 https://doi.org/10.1172/jci.insight.85817.
Morgan, L., Hawe, E., Palmen, J., Montgomery, H., Humphries, S. E., & Kitchen, N. (2005). Polymorphism of the heme oxygenase-1 gene and cerebral aneurysms. British Journal of Neurosurgery, 19(4 Aug), 317–21. https://doi.org/10.1080/02688690500305456. PMID: 16455537.
Schillinger, M., Exner, M., Mlekusch, W., Domanovits, H., Huber, K., Mannhalter, C., Wagner, O., & Minar, E. (2002). Heme oxygenase-1 gene promoter polymorphism is associated with abdominal aortic aneurysm. Thrombosis Research, 106(2 Apr), 131–6. https://doi.org/10.1016/s0049-3848(02)00100-7. PMID: 12182912.
Rodriguez, A. I., Gangopadhyay, A., Kelley, E. E., Pagano, P. J., Zuckerbraun, B. S., & Bauer, P. M. H. O.-1 (2010). and CO decrease platelet-derived growth factor-induced vascular smooth muscle cell migration via inhibition of Nox1. Arteriosclerosis, Thrombosis, and Vascular Biology, 30(1 Jan), 98–104. https://doi.org/10.1161/ATVBAHA.109.197822. Epub 2009 Oct 29. PMID: 19875720; PMCID: PMC2814251.
Liu, X., Pachori, A. S., Ward, C. A., Davis, J. P., Gnecchi, M., Kong, D., Zhang, L., Murduck, J., Yet, S. F., Perrella, M. A., Pratt, R. E., Dzau, V. J., & Melo, L. G. (2006). Heme oxygenase-1 (HO-1) inhibits postmyocardial infarct remodeling and restores ventricular function. The FASEB Journal, 20(2 Feb), 207–16. https://doi.org/10.1096/fj.05-4435com. PMID: 16449792.
Yano, Y., Ozono, R., Oishi, Y., Kambe, M., Yoshizumi, M., Ishida, T., Omura, S., Oshima, T., & Igarashi, K. (2006). Genetic ablation of the transcription repressor Bach1 leads to myocardial protection against ischemia/reperfusion in mice. Genes to Cells, 11(7 Jul), 791–803. https://doi.org/10.1111/j.1365-2443.2006.00979.x. PMID: 16824198.
Omura, S., Suzuki, H., Toyofuku, M., Ozono, R., Kohno, N., & Igarashi, K. (2005). Effects of genetic ablation of bach1 upon smooth muscle cell proliferation and atherosclerosis after cuff injury. Genes to Cells, 10(3 Mar), 277–85. https://doi.org/10.1111/j.1365-2443.2005.00832.x. PMID: 15743416.
Duckers, H. J., Boehm, M., True, A. L., Yet, S. F., San, H., Park, J. L., Clinton Webb, R., Lee, M. E., Nabel, G. J., & Nabel, E. G. (2001). Heme oxygenase-1 protects against vascular constriction and proliferation. Nature Medicine, 7(6 Jun), 693–8. https://doi.org/10.1038/89068. PMID: 11385506.
Zhao, H., Wong, R. J., Doyle, T. C., Nayak, N., Vreman, H. J., Contag, C. H., & Stevenson, D. K. (2008). Regulation of maternal and fetal hemodynamics by heme oxygenase in mice. Biology of Reproduction, 78(4 Apr), 744–51. https://doi.org/10.1095/biolreprod.107.064899. Epub 2007 Dec 19. PMID: 18094356.
Li Volti, G., Sacerdoti, D., Sangras, B., Vanella, A., Mezentsev, A., Scapagnini, G., Falck, J. R., & Abraham, N. G. (2005). Carbon monoxide signaling in promoting angiogenesis in human microvessel endothelial cells. Antioxidants Redox Signaling, 7(5–6 May–Jun), 704–10. https://doi.org/10.1089/ars.2005.7.704. PMID: 15890016.
Deramaudt, B. M., Braunstein, S., Remy, P., & Abraham, N. G. (1998). Gene transfer of human heme oxygenase into coronary endothelial cells potentially promotes angiogenesis. Journal of Cellular Biochemistry, 68(1 Jan), 121–7. 10.1002/(sici)1097-4644(19980101)68:1<121::aid-jcb12>3.0.co;2-k. PMID: 9407320.
Asahara, T., Murohara, T., Sullivan, A., Silver, M., van der Zee, R., Li, T., Witzenbichler, B., Schatteman, G., & Isner, J. M. (1997). Isolation of putative progenitor endothelial cells for angiogenesis. Science, 275(5302 Feb), 964–7. https://doi.org/10.1126/science.275.5302.964. PMID: 9020076.
Deshane, J., Chen, S., Caballero, S., Grochot-Przeczek, A., Was, H., Li Calzi, S., Lach, R., Hock, T. D., Chen, B., Hill-Kapturczak, N., Siegal, G. P., Dulak, J., Jozkowicz, A., Grant, M. B. & Agarwal, A. (2007). Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. Journal of Experimental Medicine, 204(3 Mar), 605–18. https://doi.org/10.1084/jem.20061609. Epub 2007 Mar 5. PMID: 17339405; PMCID: PMC1855437.
Dulak, J., Deshane, J., Jozkowicz, A., & Agarwal, A. (2008). Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation, 117(2 Jan), 231–41. https://doi.org/10.1161/CIRCULATIONAHA.107.698316. PMID: 18195184; PMCID: PMC5536198.
Dulak, J., Józkowicz, A., Foresti, R., Kasza, A., Frick, M., Huk, I., Green, C. J., Pachinger, O., Weidinger, F., & Motterlini, R. (2002). Heme oxygenase activity modulates vascular endothelial growth factor synthesis in vascular smooth muscle cells. Antioxidants Redox Signaling, 4(2 Apr), 229–40. https://doi.org/10.1089/152308602753666280. PMID: 12006174.
Jozkowicz, A., Huk, I., Nigisch, A., Weigel, G., Weidinger, F., & Dulak, J. (2002). Effect of prostaglandin-J(2) on VEGF synthesis depends on the induction of heme oxygenase-1. Antioxidants Redox Signaling, 4(4 Aug), 577–85. https://doi.org/10.1089/15230860260220076. PMID: 12230869.
Wang, X., Wang, Y., Lee, S. J., Kim, H. P., Choi, A. M. & Ryter, S. W. (2011). Carbon monoxide inhibits Fas activating antibody-induced apoptosis in endothelial cells. Medical Gas Research, 1(1), 8. https://doi.org/10.1186/2045-9912-1-8. Published 2011 May 18.
Rodrigo, R., & Rivera, G. (2002). Renal damage mediated by oxidative stress: a hypothesis of protective effects of red wine. Free Radical Biology & Medicine, 33(3 Aug), 409–22. https://doi.org/10.1016/s0891-5849(02)00908-5. PMID: 12126763.
Atlas, S. A. (2007). The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. Journal of Managed Care & Specialty Pharmacy, 13(8 Suppl B Oct), 9–20. https://doi.org/10.18553/jmcp.2007.13.s8-b.9. PMID: 17970613.
Ruzicka, M., Keeley, F. W., & Leenen, F. H. (1994). The renin-angiotensin system and volume overload-induced changes in cardiac collagen and elastin. Circulation, 90(4 Oct), 1989–96. https://doi.org/10.1161/01.cir.90.4.1989. PMID: 7923689.
Quan, S., Yang, L., Shnouda, S., Schwartzman, M. L., Nasjletti, A., Goodman, A. I., & Abraham, N. G. (2004). Expression of human heme oxygenase-1 in the thick ascending limb attenuates angiotensin II-mediated increase in oxidative injury. Kidney International, 65(5 May), 1628–39. https://doi.org/10.1111/j.1523-1755.2004.00562.x. PMID: 15086901.
Tracz, M. J., Juncos, J. P., Croatt, A. J., Ackerman, A. W., Grande, J. P., Knutson, K. L., Kane, G. C., Terzic, A., Griffin, M. D. & Nath, K. A. Kidney International, 72(9 Nov), 1073–80. https://doi.org/10.1038/sj.ki.5002471. Epub 2007 Aug 29. PMID: 17728706; PMCID: PMC2948968.
Liu, X. M., Chapman, G. B., Wang, H., & Durante, W. (2002). Adenovirus-mediated heme oxygenase-1 gene expression stimulates apoptosis in vascular smooth muscle cells. Circulation, 105(1 Jan), 79–84. https://doi.org/10.1161/hc0102.101369. PMID: 11772880.
Tracz, M. J., Juncos, J. P., Grande, J. P., Croatt, A. J., Ackerman, A. W., Rajagopalan, G., Knutson, K. L., Badley, A. D., Griffin, M. D., Alam, J., & Nath, K. A. (2007). Renal hemodynamic, inflammatory, and apoptotic responses to lipopolysaccharide in HO-1-/- mice. The American Journal of Pathology, 170(6 Jun), 1820–30. https://doi.org/10.2353/ajpath.2007.061093. PMID: 17525251; PMCID: PMC1899452.
Nath, K. A., Grande, J. P., Haggard, J. J., Croatt, A. J., Katusic, Z. S., Solovey, A., & Hebbel, R. P. (2001). Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. The American Journal of Pathology, 158(3 Mar), 893–903. https://doi.org/10.1016/S0002-9440(10)64037-0. PMID: 11238038; PMCID: PMC1850341.
Wiesel, P., Patel, A. P., Carvajal, I. M., Wang, Z. Y., Pellacani, A., Maemura, K., DiFonzo, N., Rennke, H. G., Layne, M. D., Yet, S. F., Lee, M. E., & Perrella, M. A. (2001). Exacerbation of chronic renovascular hypertension and acute renal failure in heme oxygenase-1-deficient mice. Circulation Research, 88(10 May), 1088–94. https://doi.org/10.1161/hh1001.091521. PMID: 11375280.
Salom, M. G., Cerón, S. N., Rodriguez, F., Lopez, B., Hernández, I., Martínez, J. G., Losa, A. M., & Fenoy, F. J. (2007). Heme oxygenase-1 induction improves ischemic renal failure: role of nitric oxide and peroxynitrite. American Journal of Physiology Heart Circulatory Physiology, 293(6 Dec), H3542–9. https://doi.org/10.1152/ajpheart.00977.2007. Epub 2007 Sep 21. PMID: 17890422.
Gelberg H. Pathophysiological mechanisms of gastrointestinal toxicity. Comprehensive Toxicology. 2018;139–178. https://doi.org/10.1016/B978-0-12-801238-3.10923-7.
Aburaya, M., Tanaka, K., Hoshino, T., Tsutsumi, S., Suzuki, K., Makise, M., Akagi, R. & Mizushima, T. (2006). Heme oxygenase-1 protects gastric mucosal cells against non-steroidal anti-inflammatory drugs. Journal of Biological Chemistry, 281(44), 33422–32. https://doi.org/10.1074/jbc.M602074200. Epub 2006 Aug 31. PMID: 16945925.
Gudis, K., & Sakamoto, C. (2005). The role of cyclooxygenase in gastric mucosal protection. Digestive Diseases and Sciences, 50(Suppl 1 Oct), S16–23. https://doi.org/10.1007/s10620-005-2802-7. PMID: 16184416.
Fiorucci, S., Antonelli, E., & Morelli, A. (2001). Mechanism of non-steroidal anti-inflammatory drug-gastropathy. Digestive and Liver Disease, 33(Suppl 2 Dec), S35–43. https://doi.org/10.1016/s1590-8658(01)80157-2. PMID: 11827361.
Attuwaybi, B. O., Kozar, R. A., Moore-Olufemi, S. D., Sato, N., Hassoun, H. T., Weisbrodt, N. W., & Moore, F. A. (2004). Heme oxygenase-1 induction by hemin protects against gut ischemia/reperfusion injury. Journal of Surgical Research, 118(1 May), 53–7. https://doi.org/10.1016/j.jss.2004.01.010. PMID: 15093717.
Scott, J. R., Cukiernik, M. A., Ott, M. C., Bihari, A., Badhwar, A., Gray, D. K., Harris, K. A., Parry, N. G., & Potter, R. F. (2009). Low-dose inhaled carbon monoxide attenuates the remote intestinal inflammatory response elicited by hindlimb ischemia-reperfusion. American Journal of Physiology Gastrointestinal and Liver Physiology, 296(1 Jan), G9–G14. https://doi.org/10.1152/ajpgi.90243.2008. Epub 2008 Oct 16. PMID: 19114681.
Otterbein, L. E., & Choi, A. M. (2000). Heme oxygenase: colors of defense against cellular stress. American Journal of Physiology Lung Cellular and Molecular Physiology, 279(6 Dec), L1029–37. https://doi.org/10.1152/ajplung.2000.279.6.L1029. PMID: 11076792.
Lee, T. S., & Chau, L. Y. (2002). Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nature Medicine, 8(3 Mar), 240–6. https://doi.org/10.1038/nm0302-240. PMID: 11875494.
Chin, B. Y., & Otterbein, L. E. (2009). Carbon monoxide is a poison… to microbes! CO as a bactericidal molecule. Current Opinion in Pharmacology, 9(4 Aug), 490–500. https://doi.org/10.1016/j.coph.2009.06.025. Epub 2009 Jul 27. PMID: 19640789.
Lee, S. S., Gao, W., Mazzola, S., Thomas, M. N., Csizmadia, E., Otterbein, L. E., Bach, F. H. & Wang, H. (2007). Heme oxygenase-1, carbon monoxide, and bilirubin induce tolerance in recipients toward islet allografts by modulating T regulatory cells. The FASEB Journal, 21(13 Nov), 3450–7. https://doi.org/10.1096/fj.07-8472com. Epub 2007 Jun 5. PMID: 17551098.
Nakao, A., Choi, A. M., & Murase, N. (2006). Protective effect of carbon monoxide in transplantation. Journal of Cellular and Molecular Medicine, 10(3 Jul-Sep), 650–71. https://doi.org/10.1111/j.1582-4934.2006.tb00426.x. PMID: 16989726; PMCID: PMC3933148.
Ceran, C., Sönmez, K., Türkyllmaz, Z., Demirogullarl, B., Dursun, A., Düzgün, E., Başaklar, A. C., & Kale, N. (2001). Effect of bilirubin in ischemia/reperfusion injury on rat small intestine. Journal of Pediatric Surgery, 36(12 Dec), 1764–7. https://doi.org/10.1053/jpsu.2001.28816. PMID: 11733902.
Nakao, A., Kimizuka, K., Stolz, D. B., Neto, J. S., Kaizu, T., Choi, A. M., Uchiyama, T., Zuckerbraun, B. S., Nalesnik, M. A., Otterbein, L. E., & Murase, N. (2003). Carbon monoxide inhalation protects rat intestinal grafts from ischemia/reperfusion injury. The American Journal of Pathology, 163(4 Oct), 1587–98. https://doi.org/10.1016/S0002-9440(10)63515-8. PMID: 14507665; PMCID: PMC1868280.
Foresti, R., Green, C. J., & Motterlini, R. (2004). Generation of bile pigments by haem oxygenase: a refined cellular strategy in response to stressful insults. Biochemical Society Symposia, 71, 177–92. https://doi.org/10.1042/bss0710177. PMID: 15777021.
Trefts, E., Gannon, M., & Wasserman, D. H. (2017). The liver. Current Biology, 27(21 Nov), R1147–R1151. https://doi.org/10.1016/j.cub.2017.09.019. PMID: 29112863; PMCID: PMC5897118.
Fraser, S. T., Midwinter, R. G., Berger, B. S., & Stocker, R. (2011). Heme Oxygenase-1: A Critical Link between Iron Metabolism, Erythropoiesis, and Development. Advances in Hematology, 2011, 473709 https://doi.org/10.1155/2011/473709. Epub 2011 Nov 20. PMID: 22162689; PMCID: PMC3226344.
Kapturczak, M. H., Wasserfall, C., Brusko, T., Campbell-Thompson, M., Ellis, T. M., Atkinson, M. A., & Agarwal, A. (2004). Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. The American Journal of Pathology, 165(3 Sep), 1045–53. https://doi.org/10.1016/S0002-9440(10)63365-2. Erratum in: Am J Pathol. 2006 Feb;168(2):714. PMID: 15331427; PMCID: PMC1618611.
Kovtunovych, G., Eckhaus, M. A., Ghosh, M. C., Ollivierre-Wilson, H., & Rouault, T. A. (2010). Dysfunction of the heme recycling system in heme oxygenase 1-deficient mice: effects on macrophage viability and tissue iron distribution. Blood, 116(26 Dec), 6054–62. https://doi.org/10.1182/blood-2010-03-272138. Epub 2010 Sep 15. PMID: 20844238; PMCID: PMC3031391.
Tsuchihashi, S., Zhai, Y., Bo, Q., Busuttil, R. W., & Kupiec-Weglinski, J. W. (2007). Heme oxygenase-1 mediated cytoprotection against liver ischemia and reperfusion injury: inhibition of type-1 interferon signaling. Transplantation, 83(12 Jun), 1628–34. https://doi.org/10.1097/01.tp.0000266917.39958.47. PMID: 17589347.
Mandal, P., Roychowdhury, S., Park, P. H., Pratt, B. T., Roger, T. & Nagy, L. E. (2010). Adiponectin and heme oxygenase-1 suppress TLR4/MyD88-independent signaling in rat Kupffer cells and in mice after chronic ethanol exposure. Journal of Immunology, 185(8 Oct), 4928–37. https://doi.org/10.4049/jimmunol.1002060. Epub 2010 Sep 22. PMID: 20861358; PMCID: PMC5085268 .
Yang, H., Zhao, L. F., Zhao, Z. F., Wang, Y., Zhao, J. J., & Zhang, L. (2012). Heme oxygenase-1 prevents liver fibrosis in rats by regulating the expression of PPARγ and NF-κB. World Journal of Gastroenterology, 18(14 Apr), 1680–8. https://doi.org/10.3748/wjg.v18.i14.1680. PMID: 22529699; PMCID: PMC3325536.
Vallabhaneni, R., Kaczorowski, D. J., Yaakovian, M. D., Rao, J., & Zuckerbraun, B. S. (2010). Heme oxygenase 1 protects against hepatic hypoxia and injury from hemorrhage via regulation of cellular respiration. Shock, 33(3 Mar), 274–81. https://doi.org/10.1097/SHK.0b013e3181b0f566. PMID: 19536046.
Zuckerbraun, B. S., Billiar, T. R., Otterbein, S. L., Kim, P. K., Liu, F., Choi, A. M., Bach, F. H., & Otterbein, L. E. (2003). Carbon monoxide protects against liver failure through nitric oxide-induced heme oxygenase 1. Journal of Experimental Medicine, 198(11 Dec), 1707–16. https://doi.org/10.1084/jem.20031003. PMID: 14657222; PMCID: PMC2194127.
Gu, Q., Wu, Q., Jin, M., Xiao, Y., Xu, J., Mao, C., Zhao, F., Zhang, Y., & Zhang, Y. (2012). Heme oxygenase-1 alleviates mouse hepatic failure through suppression of adaptive immune responses. Journal of Pharmacology and Experimental Therapeutics, 340(1 Jan), 2–10. https://doi.org/10.1124/jpet.111.186551. Epub 2011 Sep 23. PMID: 21946119.
Amersi, F., Shen, X. D., Anselmo, D., Melinek, J., Iyer, S., Southard, D. J., Katori, M., Volk, H. D., Busuttil, R. W., Buelow, R., & Kupiec-Weglinski, J. W. (2002). Ex vivo exposure to carbon monoxide prevents hepatic ischemia/reperfusion injury through p38 MAP kinase pathway. Hepatology, 35(4 Apr), 815–23. https://doi.org/10.1053/jhep.2002.32467. PMID: 11915027.
Lopez-Ojeda W., Pandey A., Alhajj M., et al. Anatomy, Skin (Integument) [Updated 2020 Nov 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441980/.
Johansen, C. (2017). Generation and culturing of primary human keratinocytes from adult skin. Journal of Visualized Experiments, 130, 56863. https://doi.org/10.3791/56863. Published 2017 Dec 22.
Nicol, N. H. (2005). Anatomy and physiology of the skin. Dermatology Nursing, 17(1 Feb), 62 PMID: 15782930.
Brenner, M., & Hearing, V. J. (2008). The protective role of melanin against UV damage in human skin. Photochemistry and Photobiology, 84(3), 539–549. https://doi.org/10.1111/j.1751-1097.2007.00226.x.
Van Harmelen, V., Reynisdottir, S., Eriksson, P., Thörne, A., Hoffstedt, J., Lönnqvist, F., & Arner, P. (1998). Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes, 47(6 Jun), 913–7. https://doi.org/10.2337/diabetes.47.6.913. PMID: 9604868.
Athar, M. (2002). Oxidative stress and experimental carcinogenesis. Indian Journal of Experimental Biology, 40(6 Jun), 656–67. PMID: 12587714.
Zhang, S., Song, C., Zhou, J., Xie, L., Meng, X., Liu, P., Cao, J., Zhang, X., Ding, W. Q., & Wu, J. (2012). Amelioration of radiation-induced skin injury by adenovirus-mediated heme oxygenase-1 (HO-1) overexpression in rats. Radiation Oncology, 7(Jan), 4 https://doi.org/10.1186/1748-717X-7-4. PMID: 22247972; PMCID: PMC3282628.
Hanselmann, C., Mauch, C., & Werner, S. (2001). Haem oxygenase-1: a novel player in cutaneous wound repair and psoriasis? Biochemical Journal, 353(Pt 3 Feb), 459–66. https://doi.org/10.1042/0264-6021:3530459. PMID: 11171041; PMCID: PMC1221590.
Clark, J. E., Green, C. J., & Motterlini, R. (1997). Involvement of the heme oxygenase-carbon monoxide pathway in keratinocyte proliferation. Biochemical and Biophysical Research Communications, 241(2 Dec), 215–20. https://doi.org/10.1006/bbrc.1997.7742. PMID: 9425252.
Listopad, J., Asadullah, K., Sievers, C., Ritter, T., Meisel, C., Sabat, R., & Döcke, W. D. (2007). Heme oxygenase-1 inhibits T cell-dependent skin inflammation and differentiation and function of antigen-presenting cells. Experimental Dermatology, 16(8 Aug), 661–70. https://doi.org/10.1111/j.1600-0625.2007.00581.x. PMID: 17620093.
Kirino, M., Kirino, Y., Takeno, M., Nagashima, Y., Takahashi, K., Kobayashi, M., Murakami, S., Hirasawa, T., Ueda, A., Aihara, M., Ikezawa, Z., & Ishigatsubo, Y. (2008). Heme oxygenase 1 attenuates the development of atopic dermatitis-like lesions in mice: implications for human disease. The Journal of Allergy and Clinical Immunology, 122(2 Aug), 290–7. https://doi.org/10.1016/j.jaci.2008.05.031. 297.e1-8Epub 2008 Jun 25. PMID: 18582925.
Zhang, B., Xie, S., Su, Z., Song, S., Xu, H., Chen, G., Cao, W., Yin, S., Gao, Q., & Wang, H. (2016). Heme oxygenase-1 induction attenuates imiquimod-induced psoriasiform inflammation by negative regulation of Stat3 signaling. Scientific Reports, 6(Feb), 21132 https://doi.org/10.1038/srep21132. PMID: 26893174; PMCID: PMC4759695.
Yasui, Y., Nakamura, M., Onda, T., Uehara, T., Murata, S., Matsui, N., Fukuishi, N., Akagi, R., Suematsu, M. & Akagi, M. (2007). Heme oxygenase-1 inhibits cytokine production by activated mast cells. Biochemical and Biophysical Research Communications, 354(2 Mar), 485–90. https://doi.org/10.1016/j.bbrc.2006.12.228. Epub 2007 Jan 10. PMID: 17234154.
Wagner, G., Lindroos-Christensen, J., Einwallner, E., Husa, J., Zapf, T. C., Lipp, K., Rauscher, S., Gröger, M., Spittler, A., Loewe, R., Gruber, F., Duvigneau, J. C., Mohr, T., Sutterlüty-Fall, H., Klinglmüller, F., Prager, G., Huppertz, B., Yun, J., Wagner, O., Esterbauer, H., & Bilban, M. (2017). HO-1 inhibits preadipocyte proliferation and differentiation at the onset of obesity via ROS dependent activation of Akt2. Scientific Reports, 7(Jan), 40881 https://doi.org/10.1038/srep40881. PMID: 28102348; PMCID: PMC5244367.
Vanella, L., Sodhi, K., Kim, D. H., Puri, N., Maheshwari, M., Hinds, T. D., Bellner, L., Goldstein, D., Peterson, S. J., Shapiro, J. I., & Abraham, N. G. (2013). Increased heme-oxygenase 1 expression in mesenchymal stem cell-derived adipocytes decreases differentiation and lipid accumulation via upregulation of the canonical Wnt signaling cascade. Stem Cell Research & Therapy, 4(2 Mar), 28 https://doi.org/10.1186/scrt176. PMID: 23497794; PMCID: PMC3706794.
Bronson, S. L., & Bale, T. L. (2016). The placenta as a mediator of stress effects on neurodevelopmental reprogramming. Neuropsychopharmacology, 41(1), 207–218. https://doi.org/10.1038/npp.2015.231.
Plitas, G., & Rudensky, A. Y. (2016). Regulatory T cells: differentiation and function. Cancer Immunology Research, 4(9), 721–725. https://doi.org/10.1158/2326-6066.CIR-16-0193.
Schumacher, A., Wafula, P. O., Teles, A., El-Mousleh, T., Linzke, N., Zenclussen, M. L., Langwisch, S., Heinze, K., Wollenberg, I., Casalis, P. A., Volk, H. D., Fest, S. & Zenclussen, A. C. (2012). Blockage of heme oxygenase-1 abrogates the protective effect of regulatory T cells on murine pregnancy and promotes the maturation of dendritic cells. PLoS One, 7(8), e42301. https://doi.org/10.1371/journal.pone.0042301. Epub 2012 Aug 10. PMID: 22900010; PMCID: PMC3416808.
Zenclussen, M. L., Linzke, N. & Schumacher, A. et al. (2015). Heme oxygenase-1 is critically involved in placentation, spiral artery remodeling, and blood pressure regulation during murine pregnancy. Frontiers in Pharmacology, 5, 291. https://doi.org/10.3389/fphar.2014.00291. Published 2015 Jan 13 .
Kaartokallio, T., Utge, S., Klemetti, M. M., Paananen, J., Pulkki, K., Romppanen, J., Tikkanen, I., Heinonen, S., Kajantie, E., Kere, J., Kivinen, K., Pouta, A., Lakkisto, P., & Laivuori, H. (2018). Fetal microsatellite in the heme oxygenase 1 promoter is associated with severe and early-onset preeclampsia. Hypertension, 71(1 Jan), 95–102. https://doi.org/10.1161/HYPERTENSIONAHA.117.10425. Epub 2017 Dec 4. PMID: 29203625.
Siasi, E., Aleyasin, A., Mowla, S. J., & Sahebkashaf, H. (2011). Study of GT-repeat expansion in Heme oxygenase-1 gene promoter as genetic cause of male infertility. Journal of Assisted Reproduction and Genetics, 28(8), 737–741. https://doi.org/10.1007/s10815-011-9574-0.
Uttara, B., et al. (2009). Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Current Neuropharmacology, 7(1), 65–74. https://doi.org/10.2174/157015909787602823.
Cobley, J. N., Fiorello, M. L., & Bailey, D. M. (2018). 13 reasons why the brain is susceptible to oxidative stress. Redox Biology, 15(May), 490–503. https://doi.org/10.1016/j.redox.2018.01.008. Epub 2018 Feb 3. PMID: 29413961; PMCID: PMC5881419.
Schipper, H. M. (2000). Heme oxygenase-1: role in brain aging and neurodegeneration. Experimental Gerontology, 35(6–7 Sep), 821–30. https://doi.org/10.1016/s0531-5565(00)00148-0. PMID: 11053673.
Funk, M., Endler, G., Schillinger, M., Mustafa, S., Hsieh, K., Exner, M., Lalouschek, W., Mannhalter, C., & Wagner, O. (2004). The effect of a promoter polymorphism in the heme oxygenase-1 gene on the risk of ischaemic cerebrovascular events: the influence of other vascular risk factors. Thrombosis Research, 113(3–4), 217–23. https://doi.org/10.1016/j.thromres.2004.03.003. PMID: 15140586.
Cuadrado, A., & Rojo, A. I. (2008). Heme oxygenase-1 as a therapeutic target in neurodegenerative diseases and brain infections. Current Pharmaceutical Design, 14(5), 429–42. https://doi.org/10.2174/138161208783597407. PMID: 18289070.
Terajima, K., Igarashi, H., Hirose, M., Matsuzawa, H., Nishizawa, M., & Nakada, T. (2008). Serial assessments of delayed encephalopathy after carbon monoxide poisoning using magnetic resonance spectroscopy and diffusion tensor imaging on 3.0T system. European Neurology, 59(1–2), 55–61. https://doi.org/10.1159/000109262. Epub 2007 Oct 4. PMID: 17917459.
Weaver, L. K. (1999). Carbon monoxide poisoning. Critical Care Clinics, 15(2 Apr), 297–317. https://doi.org/10.1016/s0749-0704(05)70056-7. viiiPMID: 10331130.
Kapitulnik, J. (2004). Bilirubin: an endogenous product of heme degradation with both cytotoxic and cytoprotective properties. Molecular Pharmacology, 66(4 Oct), 773–9. https://doi.org/10.1124/mol.104.002832. Epub 2004 Jul 21. PMID: 15269289.
Lin, S., Yin, Q., Zhong, Q., Lv, F. L., Zhou, Y., Li, J. Q., Wang, J. Z., Su, B. Y., & Yang, Q. W. (2012). Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. Journal of Neuroinflammation, 9(Mar), 46 https://doi.org/10.1186/1742-2094-9-46. PMID: 22394415; PMCID: PMC3344687.
Vasconcellos, L. R. C., Martimiano, L., Dantas, D. P., Fonseca, F. M., Mata-Santos, H., Travassos, L., Mendez-Otero, R., Bozza, M. T. & Pimentel-Coelho, P. M. (2021). Intracerebral injection of heme induces lipid peroxidation, neuroinflammation, and sensorimotor deficits. Stroke, 52(5 May), 1788–1797. https://doi.org/10.1161/STROKEAHA.120.031911. Epub 2021 Apr 8. PMID: 33827248.
Premkumar, D. R., Smith, M. A., Richey, P. L., Petersen, R. B., Castellani, R., Kutty, R. K., Wiggert, B., Perry, G., & Kalaria, R. N. (1995). Induction of heme oxygenase-1 mRNA and protein in neocortex and cerebral vessels in Alzheimer’s disease. Journal of Neurochemistry, 65(3 Sep), 1399–402. https://doi.org/10.1046/j.1471-4159.1995.65031399.x. PMID: 7543935.
Schipper, H. M., Song, W., Zukor, H., Hascalovici, J. R., & Zeligman, D. (2009). Heme oxygenase-1 and neurodegeneration: expanding frontiers of engagement. Journal of Neurochemistry, 110(2 Jul), 469–85. https://doi.org/10.1111/j.1471-4159.2009.06160.x. Epub 2009 May 11. PMID: 19457088.
Schipper, H. M., Cissé, S., & Stopa, E. G. (1995). Expression of heme oxygenase-1 in the senescent and Alzheimer-diseased brain. Annals of Neurology, 37(6 Jun), 758–68. https://doi.org/10.1002/ana.410370609. PMID: 7778849.
Castellani, R., Smith, M. A., Richey, P. L., & Perry, G. (1996). Glycoxidation and oxidative stress in Parkinson disease and diffuse Lewy body disease. Brain Research, 737(1–2 Oct), 195–200. https://doi.org/10.1016/0006-8993(96)00729-9. PMID: 8930366.
Ham, D., & Schipper, H. M. (2000). Heme oxygenase-1 induction and mitochondrial iron sequestration in astroglia exposed to amyloid peptides. Cellular and Molecular Biology (Noisy-le-grand), 46(3 May), 587–96. PMID: 10872745.
Song, W., Zukor, H., Lin, S. H., Hascalovici, J., Liberman, A., Tavitian, A., Mui, J., Vali, H., Tong, X. K., Bhardwaj, S. K., Srivastava, L. K., Hamel, E., & Schipper, H. M. (2012). Schizophrenia-like features in transgenic mice overexpressing human HO-1 in the astrocytic compartment. Journal of Neuroscience, 32(32 Aug), 10841–53. https://doi.org/10.1523/JNEUROSCI.6469-11.2012. PMID: 22875919; PMCID: PMC6621004.
Song, W., Cressatti, M., Zukor, H., Liberman, A., Galindez, C., & Schipper, H. M. (2017). Parkinsonian features in aging GFAP.HMOX1 transgenic mice overexpressing human HO-1 in the astroglial compartment. Neurobiology of Aging, 58(Oct), 163–179. https://doi.org/10.1016/j.neurobiolaging.2017.06.017. Epub 2017 Jun 28. PMID: 28746897.
Zhu, X., Perry, G., Moreira, P. I., Aliev, G., Cash, A. D., Hirai, K., & Smith, M. A. (2006). Mitochondrial abnormalities and oxidative imbalance in Alzheimer disease. Journal of Alzheimer’s Disease, 9(2 Jul), 147–53. https://doi.org/10.3233/jad-2006-9207. PMID: 16873962.
Boehning, D., Moon, C., Sharma, S., Hurt, K. J., Hester, L. D., Ronnett, G. V., Shugar, D., & Snyder, S. H. (2003). Carbon monoxide neurotransmission activated by CK2 phosphorylation of heme oxygenase-2. Neuron, 40(1 Sep), 129–37. https://doi.org/10.1016/s0896-6273(03)00596-8. PMID: 14527438.
Parfenova, H., & Leffler, C. W. (2008). Cerebroprotective functions of HO-2. Current Pharmaceutical Design, 14(5), 443–453. https://doi.org/10.2174/138161208783597380.
Takeda, A., Perry, G., Abraham, N. G., Dwyer, B. E., Kutty, R. K., Laitinen, J. T., Petersen, R. B., & Smith, M. A. (2000). Overexpression of heme oxygenase in neuronal cells, the possible interaction with Tau. Journal of Biological Chemistry, 275(8 Feb), 5395–9. https://doi.org/10.1074/jbc.275.8.5395. PMID: 10681514.
Krishnan, S., Chi, E. Y., Wood, S. J., Kendrick, B. S., Li, C., Garzon-Rodriguez, W., Wypych, J., Randolph, T. W., Narhi, L. O., Biere, A. L., Citron, M., & Carpenter, J. F. (2003). Oxidative dimer formation is the critical rate-limiting step for Parkinson’s disease alpha-synuclein fibrillogenesis. Biochemistry, 42(3 Jan), 829–37. https://doi.org/10.1021/bi026528t. PMID: 12534296.
Hahl, P., Davis, T., Washburn, C., Rogers, J. T., & Smith, A. (2013). Mechanisms of neuroprotection by hemopexin: modeling the control of heme and iron homeostasis in brain neurons in inflammatory states. Journal of Neurochemistry, 125(1), 89–101. https://doi.org/10.1111/jnc.12165.
Ronchi, C. F., Fioretto, J. R., Ferreira, A. L., Berchieri-Ronchi, C. B., Correa, C. R., Kurokawa, C. S., Carpi, M. F., Moraes, M. A. & Yeum, K. J. (2012). Biomarkers for oxidative stress in acute lung injury induced in rabbits submitted to different strategies of mechanical ventilation. Journal of Applied Physiology (1985), 112(7 Apr), 1184–90. https://doi.org/10.1152/japplphysiol.01334.2011. Epub 2012 Feb 2. PMID: 22302956.
Yamada, N., Yamaya, M., Okinaga, S., Nakayama, K., Sekizawa, K., Shibahara, S., & Sasaki, H. (2000). Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. The American Journal of Human Genetics, 66(1 Jan), 187–95. https://doi.org/10.1086/302729. Erratum in: Am J Hum Genet 2001 Jun;68(6):1542. PMID: 10631150; PMCID: PMC1288325.
He, J. Q., Ruan, J., Connett, J. E., Anthonisen, N. R., Paré, P. D., & Sandford, A. J. (2002). Antioxidant gene polymorphisms and susceptibility to a rapid decline in lung function in smokers. American Journal of Respiratory and Critical Care Medicine, 166(3 Aug), 323–8. https://doi.org/10.1164/rccm.2111059. PMID: 12153964.
Xia, Z. W., Zhong, W. W., Xu, L. Q., Sun, J. L., Shen, Q. X., Wang, J. G., Shao, J., Li, Y. Z., & Yu, S. C. (2006). Heme oxygenase-1-mediated CD4+CD25high regulatory T cells suppress allergic airway inflammation. The Journal of Immunology, 177(9 Nov), 5936–45. https://doi.org/10.4049/jimmunol.177.9.5936. PMID: 17056518.
Lee, I. T., Wang, S. W., Lee, C. W., Chang, C. C., Lin, C. C., Luo, S. F., & Yang, C. M. (2008). Lipoteichoic acid induces HO-1 expression via the TLR2/MyD88/c-Src/NADPH oxidase pathway and Nrf2 in human tracheal smooth muscle cells. The Journal of Immunology, 181(7 Oct), 5098–110. https://doi.org/10.4049/jimmunol.181.7.5098. PMID: 18802114.
Zhang, Y., Zhang, L., Wu, J., Di, C. & & Xia, Z. (2013). Heme oxygenase-1 exerts a protective role in ovalbumin-induced neutrophilic airway inflammation by inhibiting Th17 cell-mediated immune response. Journal of Biological Chemistry, 288(48 Nov), 34612–26. https://doi.org/10.1074/jbc.M113.494369. Epub 2013 Oct 4. PMID: 24097973; PMCID: PMC3843074.
Almolki, A., Taillé, C., Martin, G. F., Jose, P. J., Zedda, C., Conti, M., Megret, J., Henin, D., Aubier, M. & Boczkowski, J. (2004). Heme oxygenase attenuates allergen-induced airway inflammation and hyperreactivity in guinea pigs. American Journal of Physiology Lung Cellular and Molecular Physiology, 287(1 Jul), L26–34. https://doi.org/10.1152/ajplung.00237.2003. Epub 2004 Mar 5. PMID: 15003924 .
Otterbein, L. E., Mantell, L. L., & Choi, A. M. (1999). Carbon monoxide provides protection against hyperoxic lung injury. American Journal of Physiology, 276(4 Apr), L688–94. https://doi.org/10.1152/ajplung.1999.276.4.L688. PMID: 10198367.
Zhang, X., Shan, P., Jiang, D., Noble, P. W., Abraham, N. G., Kappas, A., & Lee, P. J. (2004). Small interfering RNA targeting heme oxygenase-1 enhances ischemia-reperfusion-induced lung apoptosis. Journal of Biological Chemistry, 279(11 Mar), 10677–84. https://doi.org/10.1074/jbc.M312941200. Epub 2003 Dec 18. PMID: 14688267.
Inoue, S., Suzuki, M., Nagashima, Y., Suzuki, S., Hashiba, T., Tsuburai, T., Ikehara, K., Matsuse, T., & Ishigatsubo, Y. (2001). Transfer of heme oxygenase 1 cDNA by a replication-deficient adenovirus enhances interleukin 10 production from alveolar macrophages that attenuates lipopolysaccharide-induced acute lung injury in mice. Human Gene Therapy, 12(8 May), 967–79. https://doi.org/10.1089/104303401750195926. PMID: 11387061.
Otterbein, L. E., Soares, M. P., Yamashita, K., & Bach, F. H. (2003). Heme oxygenase-1: unleashing the protective properties of heme. Trends in Immunology, 24(8 Aug), 449–55. https://doi.org/10.1016/s1471-4906(03)00181-9. PMID: 12909459.
Zhang, X., Shan, P., Jiang, G., Zhang, S. S., Otterbein, L. E., Fu, X. Y. & Lee, P. J. (2006). Endothelial STAT3 is essential for the protective effects of HO-1 in oxidant-induced lung injury. The FASEB Journal, 20(12 Oct), 2156–8. https://doi.org/10.1096/fj.06-5668fje. Epub 2006 Sep 13. Erratum in: FASEB J. 2007 Feb;21(2):630. PMID: 16971418.
Zhou, Z., Song, R., & Fattman, C. L., et al. (2005). Carbon monoxide suppresses bleomycin-induced lung fibrosis. The American Journal of Pathology, 166(1), 27–37. https://doi.org/10.1016/S0002-9440(10)62229-8.
Dolinay, T., Szilasi, M., Liu, M., & Choi, A. M. (2004). Inhaled carbon monoxide confers antiinflammatory effects against ventilator-induced lung injury. American Journal of Respiratory and Critical Care Medicine, 170(6 Sep), 613–20. https://doi.org/10.1164/rccm.200401-023OC. Epub 2004 May 13. PMID: 15142867.
Fanelli, V., Vlachou, A., Ghannadian, S., Simonetti, U., Slutsky, A. S., & Zhang, H. (2013). Acute respiratory distress syndrome: new definition, current and future therapeutic options. Journal of Thoracic Disease, 5(3 Jun), 326–34. https://doi.org/10.3978/j.issn.2072-1439.2013.04.05. PMID: 23825769; PMCID: PMC3698298.
Sheu, C. C., Zhai, R., Wang, Z., Gong, M. N., Tejera, P., Chen, F., Su, L., Thompson, B. T. & Christiani, D. C. (2009). Heme oxygenase-1 microsatellite polymorphism and haplotypes are associated with the development of acute respiratory distress syndrome. Intensive Care Medicine, 35(8 Aug), 1343–51. https://doi.org/10.1007/s00134-009-1504-6. Epub 2009 Jun 13. PMID: 19526221; PMCID: PMC2758618 .
Gupta, D., Ramanathan, R. P., Aggarwal, A. N., & Jindal, S. K. (2001). Assessment of factors predicting outcome of acute respiratory distress syndrome in North India. Respirology, 6(2 Jun), 125–30. https://doi.org/10.1046/j.1440-1843.2001.00324.x. PMID: 11422891.
Zhang, P. X., Murray, T. S., Villella, V. R., Ferrari, E., Esposito, S., D’Souza, A., Raia, V., Maiuri, L., Krause, D. S., Egan, M. E. & Bruscia, E. M. (2013). Reduced caveolin-1 promotes hyperinflammation due to abnormal heme oxygenase-1 localization in lipopolysaccharide-challenged macrophages with dysfunctional cystic fibrosis transmembrane conductance regulator. The Journal of Immunology, 190(10 May), 5196–206. https://doi.org/10.4049/jimmunol.1201607. Epub 2013 Apr 19. PMID: 23606537; PMCID: PMC3711148.
Dennery, P. A. (2014). Heme oxygenase in neonatal lung injury and repair. Antioxidants & Redox Signaling, 21(13 Nov), 1881–92. https://doi.org/10.1089/ars.2013.5791. Epub 2014 Feb 19. PMID: 24382006; PMCID: PMC4203111.
Namba, F., Go, H., Murphy, J. A., La, P., Yang, G., Sengupta, S., Fernando, A. P., Yohannes, M., Biswas, C., Wehrli, S. L., & Dennery, P. A. (2014). Expression level and subcellular localization of heme oxygenase-1 modulates its cytoprotective properties in response to lung injury: a mouse model. PLoS One, 9(3 Mar), e90936 https://doi.org/10.1371/journal.pone.0090936. PMID: 24599172; PMCID: PMC3944979.
Yang, G., Biswasa, C., Lin, Q. S., La, P., Namba, F., Zhuang, T., Muthu, M., & Dennery, P. A. (2013). Heme oxygenase-1 regulates postnatal lung repair after hyperoxia: role of β-catenin/hnRNPK signaling. Redox Biology, 1(1 Feb), 234–43. https://doi.org/10.1016/j.redox.2013.01.013. PMID: 24024157; PMCID: PMC3757689.
Funes, S. C., Rios, M., Fernández-Fierro, A., Covián, C., Bueno, S. M., Riedel, C. A., Mackern-Oberti, J. P., & Kalergis, A. M. (2020). Naturally derived heme-oxygenase 1 inducers and their therapeutic application to immune-mediated diseases. Frontiers in Immunology, 11(Jul), 1467. https://doi.org/10.3389/fimmu.2020.01467. PMID: 32849503; PMCID: PMC7396584.
Kinobe, R. T., Dercho, R. A., Vlahakis, J. Z., Brien, J. F., Szarek, W. A. & Nakatsu, K. (2006). Inhibition of the enzymatic activity of heme oxygenases by azole-based antifungal drugs. Journal of Pharmacology and Experimental Therapeutics, 319(1 Oct), 277–84. https://doi.org/10.1124/jpet.106.102699. Epub 2006 Jun 28. PMID: 16807364.
Pires, B. R. B., Silva, R. C. M. C., Ferreira, G. M., & Abdelhay, E. (2018). NF-kappa B: two sides of the same coin. Genes (Basel), 9(1 Jan), 24 https://doi.org/10.3390/genes9010024. PMID: 29315242; PMCID: PMC5793177.
Funding
Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior- CAPES.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Costa Silva, R.C.M., Correa, L.H.T. Heme Oxygenase 1 in Vertebrates: Friend and Foe. Cell Biochem Biophys 80, 97–113 (2022). https://doi.org/10.1007/s12013-021-01047-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-021-01047-z