Abstract
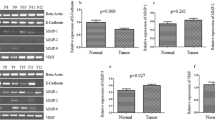
The aim of this study is to explore the expression and significance of urokinase-type plasminogen activator (u-PA) and vascular endothelial growth factor (VEGF) in gastric cancer, providing a novel insight for the diagnosis and treatment of gastric cancer. The gastric cancer specimens, which were excised from 87 patients and confirmed during July, 2012–July, 2014, were selected as observation group, and the normal tissue next to the tumor (more than 5 cm from the edge of the tumor) from 45 patients were randomly selected as control. u-PA and VEGF were detected by immunohistochemistry for the analysis of the correlation of u-PA and VEGF in two groups. The positive rates of u-PA and VEGF in gastric cancer tissue were 81.61 and 79.31 %, respectively, which were 6.67 and 8.89 % in the control group, respectively. The positive rates in the observation group were obviously higher than those in the control group, and the difference was statistically significant (P < 0.05). Among the 87 gastric cancer tissue samples from the observation group, the positive rates of u-PA and VEGF in the gastric cancer with poor differentiation, lymphatic metastasis, invasion up to serosal layer, and TNM stage III + IV were obviously higher than those in the gastric cancer with high differentiation, non-lymphatic metastasis, invasion not up to the serosal layer, and TNM stage I + II, and the difference was statistically significant (P < 0.05). Among the 87 gastric cancer tissue samples from the observation group, u-PA and VEGF were found to be positive in 60 cases and negative in 7 cases. By comparing the two groups, u-PA and VEGF were positively correlated in gastric cancer tissue (P < 0.05). u-PA and VEGF were highly expressed in gastric cancer tissue, which could be used as the molecular biological indicators to predict the invasion and metastasis potential of gastric cancer. The combination of two factors plays a guiding role in early diagnosis and treatment of gastric cancer.
Similar content being viewed by others
References
Villanueva, M. T. (2014). Therapeutics: Gastric cancer gets a red carpet treatment. Nature Reviews Cancer, 14(10), 647.
Liang, Q., et al. (2014). Integrative identification of Epstein–Barr virus-associated mutations and epigenetic alterations in gastric cancer. Gastroenterology, 147(6), 1350–1362.
Chen, X., et al. (2014). Upregulation of miR-513b inhibits cell proliferation, migration, and promotes apoptosis by targeting high mobility group-box 3 protein in gastric cancer. Tumor Biology, 35(11), 11081–11089.
Lin, L., et al. (2015). MACC1 supports human gastric cancer growth under metabolic stress by enhancing the Warburg effect. Oncogene, 34(21), 2700–2710.
Ding, Y., et al. (2012). u-PA inhibitor amiloride suppresses peritoneal metastasis in gastric cancer. World Journal of Surgical Oncology, 10, 270.
Roomi, M. W., et al. (2013). Modulation of u-PA, MMPs and their inhibitors by a novel nutrient mixture in human lung cancer and mesothelioma cell lines. International Journal of Oncology, 42(6), 1883–1889.
Weiss, T. W., et al. (2011). Oncostatin M and IL-6 induce u-PA and VEGF in prostate cancer cells and correlate in vivo. Anticancer Research, 31(10), 3273–3278.
Ho, Y. T., et al. (2009). Berberine suppresses in vitro migration and invasion of human SCC-4 tongue squamous cancer cells through the inhibitions of FAK, IKK, NF-kappaB, u-PA and MMP-2 and -9. Cancer Letters, 279(2), 155–162.
Kigure, W., et al. (2013). The association of VEGF-C expression with tumor lymphatic vessel density and lymph node metastasis in patients with gastric cancer and gastrointestinal stromal tumor. Hepato Gastroenterology, 60(122), 277–280.
Miyake, K., et al. (2012). The novel hypoxic cytotoxin, TX-2098 has antitumor effect in pancreatic cancer; possible mechanism through inhibiting VEGF and hypoxia inducible factor-1alpha targeted gene expression. Experimental Cell Research, 318(13), 1554–1563.
Zhang, T., et al. (2011). Down-regulation of MiR-206 promotes proliferation and invasion of laryngeal cancer by regulating VEGF expression. Anticancer Research, 31(11), 3859–3863.
Zhang, L., et al. (2012). Endostar down-regulates HIF-1 and VEGF expression and enhances the radioresponse to human lung adenocarcinoma cancer cells. Molecular Biology Reports, 39(1), 89–95.
Salomon-Perzynska, M., et al. (2014). VEGF-targeted therapy for the treatment of cervical cancer: Literature review. Ginekologia Polska, 85(6), 461–465.
Koessler, T., Roth, A., & Cacheux, W. (2014). Early gastric cancer: epidemiology, diagnostic and management. Revue Médicale Suisse, 10(431), 1118–1122.
Shridhar, R., et al. (2013). Increased survival associated with surgery and radiation therapy in metastatic gastric cancer: A surveillance, epidemiology, and end results database analysis. Cancer, 119(9), 1636–1642.
Lin, Y., et al. (2011). Comparative epidemiology of gastric cancer between Japan and China. World Journal of Gastroenterology, 17(39), 4421–4428.
de la Torre Bravo, A., et al. (2010). Diagnosis and treatment guideline of gastric cancer. Epidemiology, risk factors, histologic varieties and natural history. Revista de Gastroenterologia de Mexico, 75(2), 237–239.
Peddireddy, V., et al. (2014). Genetic instability in peripheral lymphocytes as biological marker for non-small cell lung cancer patients in the South Indian state of Andhra Pradesh. International Journal of Biological Marker, 29(4)e, 345–353.
Rasmussen, L., et al. (2013). Tissue inhibitor of metalloproteinases-1 as a biological marker in colorectal cancer: influence of smoking on plasma levels? International Journal of Biological Markers, 28(2), 226–230.
Fountzilas, G., et al. (2011). Paclitaxel and bevacizumab as first line combined treatment in patients with metastatic breast cancer: the Hellenic Cooperative Oncology Group experience with biological marker evaluation. Anticancer Research, 31(9), 3007–3018.
Xu, T. P., et al. (2014). Decreased expression of the long non-coding RNA FENDRR is associated with poor prognosis in gastric cancer and FENDRR regulates gastric cancer cell metastasis by affecting fibronectin1 expression. Journal of Hematology and Oncology, 7, 63.
Lin, G., et al. (2014). Overexpression of muscarinic receptor 3 promotes metastasis and predicts poor prognosis in non-small-cell lung cancer. Journal of Thoracic Oncology, 9(2), 170–178.
Lou, X., et al. (2013). Decreased expression of microRNA-625 is associated with tumor metastasis and poor prognosis in patients with colorectal cancer. Journal of Surgical Oncology, 108(4), 230–235.
Chen, Q. Y., et al. (2012). Expression of ezrin in human non-small cell lung cancer and its relationship with metastasis and prognosis. Zhonghua Zhong Liu Za Zhi, 34(6), 436–440.
Zhu, X. J., et al. (2014). Novel tumor-suppressor gene epidermal growth factor-containing fibulin-like extracellular matrix protein 1 is epigenetically silenced and associated with invasion and metastasis in human gastric cancer. Molecular Medicine Reports, 9(6), 2283–2292.
Oskarsson, T. (2013). Extracellular matrix components in breast cancer progression and metastasis. Breast, 22(Suppl 2), S66–S72.
van Horssen, R., et al. (2013). Cancer cell metabolism regulates extracellular matrix degradation by invadopodia. European Journal of Cell Biology, 92(3), 113–121.
Lescarbeau, R. M., et al. (2012). In vitro model of metastasis to bone marrow mediates prostate cancer castration resistant growth through paracrine and extracellular matrix factors. PLoS ONE, 7(8), e40372.
Ming, M., et al. (2007). The role of p38 MAPK in gastrin-induced u-PA expression in human colon cancer cells. Zhonghua Zhong Liu Za Zhi, 29(1), 4–8.
Chen, P. N., et al. (2006). Black rice anthocyanins inhibit cancer cells invasion via repressions of MMPs and u-PA expression. Chemico Biological Interactions, 163(3), 218–229.
Cheng, D., Kong, H., & Li, Y. (2013). Prognostic values of VEGF and IL-8 in malignant pleural effusion in patients with lung cancer. Biomarkers, 18(5), 386–390.
Fakih, M. (2013). The evolving role of VEGF-targeted therapies in the treatment of metastatic colorectal cancer. Expert Review of Anticancer Therapy, 13(4), 427–438.
Marton, I., et al. (2012). Immunohistochemical expression and prognostic significance of HIF-1alpha and VEGF-C in neuroendocrine breast cancer. Anticancer Research, 32(12), 5227–5232.
Conflict of interest
The authors have not declared any conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Xw., Gao, F., Chen, Yj. et al. The Clinical Study of Urokinase-Type Plasminogen Activator and Vascular Endothelial Growth Factor in Gastric Cancer. Cell Biochem Biophys 72, 649–652 (2015). https://doi.org/10.1007/s12013-014-0356-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0356-z