Abstract
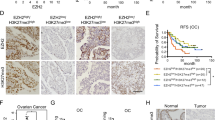
Epithelial ovarian cancer (EOC) is the second leading cause of death from gynecological malignancies worldwide. Enhancer of zeste homology 2 (EZH2), participating in gene expression silencing by trimethylating histone 3 lysine 27 (H3K27me3), is often up-regulated in EOC. ARHI, an imprinted tumor-suppressor gene, is markedly down-regulated or even undetectable in the majority of EOC. To explore the correlation between EZH2 and ARHI expression in EOC as well as the possible mechanism of EZH2–ARH1 interaction. We used immunohistochemical staining to evaluate the expression of EZH2 and ARHI in EOC and normal ovarian tissue specimens; western blotting, shRNA, and chromatin immunoprecipitation were used to study the expression correlation of EZH2 and ARHI in EOC and normal ovarian epithelial cells and to further explore the mechanism of EZH2 regulation of ARHI expression. Cell viability assay was used to evaluate the influence of these two genes on cell survival. (1) The expression of EZH2 inversely correlated with ARHI expression levels and predicted shorter overall survival in EOC patients; (2) EZH2 promoted repression of ARHI by catalyzing trimethylation on H3K27; (3) ARHI was synergistically silenced by DNA methylation and histone modification; and (4) DZNep, an inhibitor of EZH2, significantly reduced survival rate of EOC cells by restoring ARHI expression. EZH2-″induced H3K27me3 is associated with epigenetic repression of the ARHI tumor-suppressor gene in EOC. Suppression of EZH2 by DZNep, as a way of restoring the expression of ARHI, could be a potential treatment modality to EOC.




Similar content being viewed by others
References
Rescigno, P., Cerillo, I., Ruocco, R., Condello, C., De Placido, S., & Pensabene, M. (2013). New hypothesis on pathogenesis of ovarian cancer lead to future tailored approaches. BioMed Research International, 2013, 852839.
Chase, A., & Cross, N. C. (2011). Aberrations of EZH2 in cancer. Clinical Cancer Research, 17, 2613–2618.
Li, H., & Zhang, R. (2013). Role of EZH2 in epithelial ovarian cancer: from biological insights to therapeutic target. Frontiers in Oncology, 3, 47.
Lu, C., Han, H. D., Mangala, L. S., Ali-Fehmi, R., Newton, C. S., Ozbun, L., et al. (2010). Regulation of tumor angiogenesis by EZH2. Cancer Cell, 18, 185–197.
Seward, S., Semaan, A., Qazi, A. M., Gruzdyn, O. V., Chamala, S., Bryant, C. C., et al. (2013). EZH2 blockade by RNA interference inhibits growth of ovarian cancer by facilitating re-expression of p21(waf1/cip1) and by inhibiting mutant p53. Cancer Letters, 336, 53–60.
Peng, H., Xu, F., Pershad, R., Hunt, K. K., Frazier, M. L., Berchuck, A., et al. (2000). ARHI is the center of allelic deletion on chromosome 1p31 in ovarian and breast cancers. International Journal of Cancer, 86, 690–694.
Yu, Y., Xu, F., Peng, H., Fang, X., Zhao, S., Li, Y., et al. (1999). NOEY2 (ARHI), an imprinted putative tumor suppressor gene in ovarian and breast carcinomas. Proceedings of the National Academy of Sciences of the United States of America, 96, 214–219.
Lu, Z., Luo, R. Z., Lu, Y., Zhang, X., Yu, Q., Khare, S., et al. (2008). The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. The Journal of Clinical Investigation, 118, 3917–3929.
Lu, Z., Luo, R. Z., Peng, H., Rosen, D. G., Atkinson, E. N., Warneke, C., et al. (2006). Transcriptional and posttranscriptional down-regulation of the imprinted tumor suppressor gene ARHI (DRAS3) in ovarian cancer. Clinical Cancer Research, 12, 2404–2413.
Feng, W., Marquez, R. T., Lu, Z., Liu, J., Lu, K. H., Issa, J. P., et al. (2008). Imprinted tumor suppressor genes ARHI and PEG3 are the most frequently down-regulated in human ovarian cancers by loss of heterozygosity and promoter methylation. Cancer, 112, 1489–1502.
Yuan, J., Luo, R. Z., Fujii, S., Wang, L., Hu, W., Andreeff, M., et al. (2003). Aberrant methylation and silencing of ARHI, an imprinted tumor suppressor gene in which the function is lost in breast cancers. Cancer Research, 63, 4174–4180.
Fujii, S., Luo, R. Z., Yuan, J., Kadota, M., Oshimura, M., Dent, S. R., et al. (2003). Reactivation of the silenced and imprinted alleles of ARHI is associated with increased histone H3 acetylation and decreased histone H3 lysine 9 methylation. Human Molecular Genetics, 12, 1791–1800.
Lu, Z., Luo, R. Z., Peng, H., Huang, M., Nishmoto, A., Hunt, K. K., et al. (2006). E2F-HDAC complexes negatively regulate the tumor suppressor gene ARHI in breast cancer. Oncogene, 25, 230–239.
Li, H., Bitler, B. G., Vathipadiekal, V., Maradeo, M. E., Slifker, M., Creasy, C. L., et al. (2012). ALDH1A1 is a novel EZH2 target gene in epithelial ovarian cancer identified by genome-wide approaches. Cancer Prevention Research (Philadelphia, PA.), 5, 484–491.
Chiba, T., Suzuki, E., Negishi, M., Saraya, A., Miyagi, S., Konuma, T., et al. (2012). 3-Deazaneplanocin A is a promising therapeutic agent for the eradication of tumor-initiating hepatocellular carcinoma cells. International Journal of Cancer, 130, 2557–2567.
Tan, J., Yang, X., Zhuang, L., Jiang, X., Chen, W., Lee, P. L., et al. (2007). Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes & Development, 21, 1050–1063.
Fiskus, W., Wang, Y., Sreekumar, A., Buckley, K. M., Shi, H., Jillella, A., et al. (2009). Combined epigenetic therapy with the histone methyltransferase EZH2 inhibitor 3-deazaneplanocin A and the histone deacetylase inhibitor panobinostat against human AML cells. Blood, 114, 2733–2743.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No. is 81172482), the Science and Technology Department of Shandong Province (Grant No. is 2008BS03044), Natural Science Foundation of Shandong Province (Grant No. is ZR2013HQ060) and Postdoctoral Science Foundation of China (Grant No. is 2012M520201).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.

Supplemental Fig. 1
Confiration of EZH2 knock-down efficiency in EOC cell lines. SKOV3 and OVCAR3 cells were infected with a lentiviral vector encoding control or shEZH2. Protein level of EZH2 was evaluated by immunoblotting. (JPEG 38 kb)
Rights and permissions
About this article
Cite this article
Fu, Y., Chen, J., Pang, B. et al. EZH2-Induced H3K27me3 is Associated with Epigenetic Repression of the ARHI Tumor-Suppressor Gene in Ovarian Cancer. Cell Biochem Biophys 71, 105–112 (2015). https://doi.org/10.1007/s12013-014-0168-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0168-1