Abstract
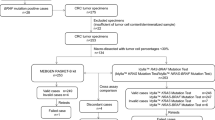
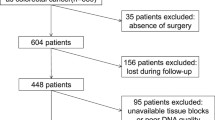
We determined frequency/types of K-ras mutations in colorectal/lung cancer. ADx-K-ras kit (real-time/double-loop probe PCR) was used to detect somatic tumor gene mutations compared with Sanger DNA sequencing using 583 colorectal and 244 lung cancer paraffin-embedded clinical samples. Genomic DNA was used in both methods; mutation rates at codons 12/13 and frequency of each mutation were detected and compared. The data show that 91.4% colorectal and 59.0% lung carcinoma samples were detected conclusively by DNA sequencing, whereas 100% colorectal and lung samples were detected by ADx-K-ras kit. K-ras gene mutations were detected in 32.9–27.4% colorectal samples using kit and sequencing methods, respectively. Whereas 10.6–8.3% lung cancer samples were positively detected by kit and sequencing methods, respectively. Notably, 172/677 showed mutations and 467/677 showed wild type by both methods; 38 samples showed mutations with kit but wild type with sequencing. Mutations in colorectal samples were as follows: GGT → GAT/codon-12 (35.1%); GGC → GAC/codon-13 (26.6%); GGT → GTT/codon-12 (18.2%); and GGT → GCT/codon-12 (1.6%). Mutations in lung samples were as follows: GGT > GTT/codon-12 (40.9%) and GGT > GCT/codon-12 (4.5%). In conclusion, K-ras mutations involved 32.2% colorectal and 10.6% lung samples among this cohort. ADx-K-ras real-time PCR showed higher detection rates (P < 0.05). The kit method has good clinical applicability as it is simple, fast, less prone to contamination and hence can be used effectively and reliably for clinical screening of somatic tumor gene mutations.


Similar content being viewed by others
References
Papadopoulos, N., Kinzler, K. W., & Vogelstein, B. (2006). The role of companion diagnostics in the development and use of mutation-targeted cancer therapies. Nature Biotechnology, 24, 985–995.
Dong, Q. G., Huang, J. S., & Huang, J. (2005). Advances in targeted therapy of lung cancer and EGFR gene mutations in lung cancer in China. Cancer, 25, 325–634.
Di Fiore, F., Blanchard, F., Charbonnier, F., Le Pessot, F., Lamy, A., Galais, M. P., et al. (2007). Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. British Journal of Cancer, 96, 1166–1169.
Linardou, H., Dahabreh, I. J., Kanaloupiti, D., Siannis, F., Bafaloukos, D., Kosmidis, P., et al. (2008). Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: A systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet, 9, 962–972.
Bria, E., Cuppone, F., & Di Maio, M. (2009). Cetuximab for metastatic colorectal cancer. The New England Journal of Medicine, 361, 95–96.
Mok, T. S., Wu, Y. L., Thongprasert, S., Yang, C. H., Chu, D. T., Saijo, N., et al. (2009). Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. The New England Journal of Medicine, 361, 947–957.
Gralow, J., Ozols, R. F., Bajorin, D. F., Cheson, B. D., Sandler, H. M., Winer, E. P., et al. (2008). Clinical cancer advances 2007: Major research advances in cancer treatment, prevention, and screening–a report from the American society of clinical oncology. Journal of Clinical Oncology, 26, 313–325.
Kimura, H., Kasahara, K., Kawaishi, M., Kunitoh, H., Tamura, T., Holloway, B., et al. (2006). Detection of epidermal growth factor receptor mutations in serum as a predictor of the response to gefitinib in patients with non-small-cell lung cancer. Clinical Cancer Research, 12, 3915–3921.
Sharma, S. V., Bell, D. W., Settleman, J., & Haber, D. A. (2007). Epidermal growth factor receptor mutations in lung cancer. Nature Reviews Cancer, 7, 169–181.
Sequist, L. V., & Lynch, T. J. (2008). EGFR tyrosine kinase inhibitors in lung cancer: An evolving story. Annual Review of Medicine, 59, 429–442.
Mackey, J., McLeod, D., Ragaz, J., Gelmon, K., Verma, S., Pritchard, K., et al. (2009). Adjuvant targeted therapy in early breast cancer. Cancer, 115, 1154–1168.
Hilger, R. A., Scheulen, M. E., & Strumberg, D. (2002). The Ras-Raf-MEK-ERK pathway in the treatment of cancer. Onkologie, 25, 511–518.
Kopreski, M. S., Benko, F. A., Kwee, C., Leitzel, K. E., Eskander, E., Lipton, A., et al. (1997). Detection of mutant K-ras DNA in plasma or serum of patients with colorectal cancer. British Journal of Cancer, 76, 1293–1299.
Barnard, R., Futo, V., Pecheniuk, N., Slattery, M., & Walsh, T. (1998). PCR bias toward the wild-type k-ras and p53 sequences: Implications for PCR detection of mutations and cancer diagnosis. Biotechniques, 25, 684–691.
Tada, M., Ohashi, M., Shiratori, Y., Okudaira, T., Komatsu, Y., Kawabe, T., et al. (1996). Analysis of K-ras gene mutation in hyperplastic duct cells of the pancreas without pancreatic disease. Gastroenterology, 110, 227–231.
Theodor, L., Melzer, E., Sologov, M., Idelman, G., Friedman, E., & Bar-Meir, S. (1999). Detection of pancreatic carcinoma: Diagnostic value of K-ras mutations in circulating DNA from serum. Digestive Diseases and Sciences, 44, 2014–2019.
Yamada, T., Nakamori, S., Ohzato, H., Oshima, S., Aoki, T., Higaki, N., et al. (1998). Detection of K-ras gene mutations in plasma DNA of patients with pancreatic adenocarcinoma: Correlation with clinicopathological features. Clinical Cancer Research, 4, 1527–1532.
Chen, H. J., Li, H. B., Li, S. M., Lu, B. W., Chen, J. F., & Liu, Y. L. (2010). The relationship between k-ras gene mutations and colorectal cancer. Chinese Clinical Cancer Research, 22, 461–463.
Rivero, E. R., Neves, A. C., Silva-Valenzuela, M. G., Sousa, S. O., & Nunes, F. D. (2006). Simple salting-out method for DNA extraction from formalin-fixed, paraffin-embedded tissues. Pathology, Research and Practice, 202, 523–529.
Tan, Z. Y., Ding, M., & Wang, B. J. (2006). Formalin-fixed paraffin-embedded tissue DNA extraction. Journal of Forensic Medicine, 22, 455–458.
Weichert, W., Schewe, C., Lehmann, A., Sers, C., Denkert, C., Budczies, J., et al. (2010). KRAS genotyping of paraffin-embedded colorectal cancer tissue in routine diagnostics. Journal of Molecular Diagnostics, 12, 35–42.
Franklin, W. A., Haney, J., Sugita, M., Bemis, L., Jimeno, A., & Messersmith, W. A. (2010). KRAS mutation- comparison of testing methods and tissue sampling techniques in colon cancer. Journal of Molecular Diagnostics, 12, 43–50.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, H., Zheng, X., Ji, T. et al. Comparative Screening of K-ras Mutations in Colorectal Cancer and Lung Cancer Patients Using a Novel Real-Time PCR with ADx-K-ras Kit and Sanger DNA Sequencing. Cell Biochem Biophys 62, 415–420 (2012). https://doi.org/10.1007/s12013-011-9318-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-011-9318-x