Abstract
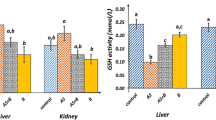
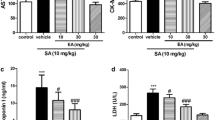
NaAsO2 is known as a harmful pollutant all over the world, and many chronic heart diseases can be attributed to its prolonged exposure in NaAsO2-contaminated water. Therefore, considering the anti-inflammatory and antioxidant effects of betaine (BET), in this study, our team investigated the cardioprotective effects of this phytochemical agent on sodium arsenite (NaAsO2)-induced cardiotoxicity. Forty male mice were randomly divided into 4 groups: (I) Control; (II) BET (500 mg/kg); (III) NaAsO2 (50 ppm); and (IV) NaAsO2 + BET. NaAsO2 was given to the animals for 8 weeks, but BET was given in the last two weeks. After decapitation, inflammatory factors and biochemical parameters were measured, and Western blot analyses were performed. BET decrease the activity level of alanine aspartate aminotransferase, creatine kinase MB, thiobarbituric acid reactive substances level, inflammatory factors (tumor necrosis factor-α) content, and nuclear factor kappa B expression. Furthermore, BET increased cardiac total thiol and activity levels of catalase, superoxide dismutase, and glutathione peroxidase and nuclear factor erythroid-2 expression. Hence, the administration of BET ameliorated the deleterious effects stemming from the imbalance of oxidative and antioxidant pathways and histopathological alterations observed in NaAsO2-intoxicated mice, thereby attenuating oxidative stress-induced damage and inflammation.






Similar content being viewed by others
Data Availability
The datasets used and analyzed during the current study are available.
References
Moon, K., Guallar, E., & Navas-Acien, A. (2012). Arsenic exposure and cardiovascular disease: An updated systematic review. Current Atherosclerosis Reports, 14, 542–555.
Jiang, X., & Yan, M. (2021). Surgical treatment for improved 1-year survival in patients with primary cardiac sarcoma. Anatolian Journal of Cardiology, 25, 796.
Zhu, Y., Huang, R., Wu, Z., Song, S., Cheng, L., & Zhu, R. (2021). Deep learning-based predictive identification of neural stem cell differentiation. Nature Communications, 12, 2614.
Xiaolong, J., Jianhang, G., Jingyuan, T., Ke, M., & Yanqi, L. (2023). Research progress on degradation methods and product properties of plant polysaccharides. Journal of Light Industry, 38, 1.
Mumford, J. L., Wu, K., Xia, Y., Kwok, R., Yang, Z., Foster, J., & Sanders, W. E., Jr. (2007). Chronic arsenic exposure and cardiac repolarization abnormalities with QT interval prolongation in a population-based study. Environmental Health Perspectives, 115, 690–694.
Alissa, E. M., & Ferns, G. A. (2011). Heavy metal poisoning and cardiovascular disease. Journal of Toxicology, 2011, 1.
Mathews, V., Paul, M., Abhilash, M., Manju, A., Abhilash, S., & Nair, R. H. (2013). Myocardial toxicity of acute promyelocytic leukaemia drug-arsenic trioxide. European Review in Medicine and Pharmacological Science, 17, 34–38.
Zhou, L., Liu, Y., Sun, H., Li, H., Zhang, Z., & Hao, P. (2022). Usefulness of enzyme-free and enzyme-resistant detection of complement component 5 to evaluate acute myocardial infarction. Sensors and Actuators B: Chemical, 369, 132315.
Fu, Q., Chen, R., Ding, Y., Xu, S., Huang, C., He, B., Jiang, T., Zeng, B., Bao, M., & Li, S. (2023). Sodium intake and the risk of various types of cardiovascular diseases: A Mendelian randomization study. Frontiers in Nutrition, 10, 1.
Yang, W., Ding, N., Luo, R., Zhang, Q., Li, Z., Zhao, F., Zhang, S., Zhang, X., Zhou, T., & Wang, H. (2023). Exosomes from young healthy human plasma promote functional recovery from intracerebral hemorrhage via counteracting ferroptotic injury. Bioactive Materials, 27, 1–14.
Yu, Y., Wang, L., Ni, S., Li, D., Liu, J., Chu, H. Y., Zhang, N., Sun, M., Li, N., & Ren, Q. (2022). Targeting loop3 of sclerostin preserves its cardiovascular protective action and promotes bone formation. Nature Communications, 13, 4241.
Samanta, J., Mondal, A., Saha, S., Chakraborty, S., & Sengupta, A. (2020). Oleic acid protects from arsenic-induced cardiac hypertrophy via AMPK/FoxO/NFATc3 pathway. Cardiovascular Toxicology, 20, 261–280.
Klaassen, C. D., & Amdur, M. O. (2013). Casarett and Doull’s toxicology: The basic science of poisons. McGraw-Hill.
Moon, K. A., Guallar, E., Umans, J. G., Devereux, R. B., Best, L. G., Francesconi, K. A., Goessler, W., Pollak, J., Silbergeld, E. K., & Howard, B. V. (2013). Association between exposure to low to moderate arsenic levels and incident cardiovascular disease: A prospective cohort study. Annals of Internal Medicine, 159, 649–659.
Alamolhodaei, N. S., Shirani, K., & Karimi, G. (2015). Arsenic cardiotoxicity: An overview. Environmental Toxicology and Pharmacology, 40, 1005–1014.
Jomova, K., Jenisova, Z., Feszterova, M., Baros, S., Liska, J., Hudecova, D., Rhodes, C. J., & Valko, M. (2011). Arsenic: Toxicity, oxidative stress and human disease. Journal of Applied Toxicology, 31, 95–107.
Kuzu, M., Kandemir, F. M., Yıldırım, S., Çağlayan, C., & Küçükler, S. (2021). Attenuation of sodium arsenite-induced cardiotoxicity and neurotoxicity with the antioxidant, anti-inflammatory, and antiapoptotic effects of hesperidin. Environmental Science and Pollution Research, 28, 10818–10831.
De Zwart, F., Slow, S., Payne, R., Lever, M., George, P., Gerrard, J., & Chambers, S. (2003). Glycine betaine and glycine betaine analogues in common foods. Food Chemistry, 83, 197–204.
Sakamoto, A., Nishimura, Y., Ono, H., & Sakura, N. (2002). Betaine and homocysteine concentrations in foods. Pediatrics International, 44, 409–413.
Craig, S. A. (2004). Betaine in human nutrition. The American Journal of Clinical Nutrition, 80, 539–549.
Alirezaei, M., Jelodar, G., Niknam, P., Ghayemi, Z., & Nazifi, S. (2011). Betaine prevents ethanol-induced oxidative stress and reduces total homocysteine in the rat cerebellum. Journal of Physiology and Biochemistry, 67, 605–612.
Al-Hafyan, S., Asoodeh, A., Baghshani, H., & Salari, L. E. (2023). Ameliorative potential of betaine against arsenite-induced hepatotoxicity and nephrotoxicity. Comparative Clinical Pathology, 1, 1–8.
Jung, Y. S., Kim, S. J., Kwon, D. Y., Ahn, C. W., Kim, Y. S., Choi, D. W., & Kim, Y. C. (2013). Alleviation of alcoholic liver injury by betaine involves an enhancement of antioxidant defense via regulation of sulfur amino acid metabolism. Food and Chemical Toxicology, 62, 292–298.
Day, C. R., & Kempson, S. A. (2016). Betaine chemistry, roles, and potential use in liver disease. Biochimica et Biophysica Acta (BBA)-General Subjects, 1860, 1098–1106.
Murillo-Fuentes, M. L., Artillo, R., Ubeda, N., Varela-Moreiras, G., Murillo, M. L., & Carreras, O. (2005). Hepatic S-adenosylmethionine after maternal alcohol exposure on offspring rats. Addiction Biology, 10, 139–144.
van der Veen, S., Hain, T., Wouters, J. A., Hossain, H., de Vos, W. M., Abee, T., Chakraborty, T., & Wells-Bennik, M. H. (2007). The heat-shock response of Listeria monocytogenes comprises genes involved in heat shock, cell division, cell wall synthesis, and the SOS response. Microbiology, 153, 3593–3607.
Burg, M. B. (1995). Molecular basis of osmotic regulation. American Journal of Physiology-Renal Physiology, 268, F983–F996.
Qiu, H., Chen, X., Luo, Z., Zhao, L., Zhang, T., Yang, N., Long, X., Xie, H., Liu, J., & Xu, W. (2018). Inhibition of endogenous hydrogen sulfide production exacerbates the inflammatory response during urine-derived sepsis-induced kidney injury. Experimental and Therapeutic Medicine, 16, 2851–2858.
Bao, M.-H., Luo, H.-Q., Chen, L.-H., Tang, L., Ma, K.-F., Xiang, J., Dong, L.-P., Zeng, J., Li, G.-Y., & Li, J.-M. (2016). Impact of high fat diet on long non-coding RNAs and messenger RNAs expression in the aortas of ApoE(−/−) mice. Scientific Reports, 6, 34161.
Zhou, Y., Sun, X., Yang, G., Ding, N., Pan, X., Zhong, A., Guo, T., Peng, Z., & Chai, X. (2023). Sex-specific differences in the association between steps per day and all-cause mortality among a cohort of adult patients from the United States with congestive heart failure. Heart & Lung, 62, 175–179.
Tang, L., Wang, Y., Xiang, J., Yang, D., Zhang, Y., Xiang, Q., & Li, J. (2023). lncRNA and circRNA expression profiles in the hippocampus of Aβ 25–35-induced AD mice treated with tripterygium glycoside. Experimental and Therapeutic Medicine, 26, 1–14.
Feng, S., Liu, W., Deng, S., Song, G., Zhou, J., Zheng, Z., & Song, Z. (2022). An atopic dermatitis-like mouse model by alternate epicutaneous application of dinitrofluorobenzene and an extract of dermatophagoides farinae. Frontiers in Medicine, 9, 843230.
Sternbach, S., & McDonough, J. (2023). Betaine as a neuroprotective therapy in multiple sclerosis (Chapter 24). In C. R. Martin, V. B. Patel, & V. R. Preedy (Eds.), Treatments, nutraceuticals, supplements, and herbal medicine in neurological disorders (pp. 443–452). Academic Press.
Ashtary-Larky, D., Bagheri, R., Ghanavati, M., Asbaghi, O., Tinsley, G. M., Mombaini, D., Kooti, W., Kashkooli, S., & Wong, A. (2022). Effects of betaine supplementation on cardiovascular markers: A systematic review and meta-analysis. Critical Reviews in Food Science and Nutrition, 62, 6516–6533.
Zhao, G., He, F., Wu, C., Li, P., Li, N., Deng, J., Zhu, G., Ren, W., & Peng, Y. (2018). Betaine in inflammation: Mechanistic aspects and applications. Frontiers in Immunology, 9, 1070.
Ganesan, B., Buddhan, S., Anandan, R., Sivakumar, R., & AnbinEzhilan, R. (2010). Antioxidant defense of betaine against isoprenaline-induced myocardial infarction in rats. Molecular Biology Reports, 37, 1319–1327.
Wu, M.-M., Chiou, H.-Y., Hsueh, Y.-M., Hong, C.-T., Su, C.-L., Chang, S.-F., Huang, W.-L., Wang, H.-T., Wang, Y.-H., Hsieh, Y.-C., & Chen, C.-J. (2006). Effect of plasma homocysteine level and urinary monomethylarsonic acid on the risk of arsenic-associated carotid atherosclerosis. Toxicology and Applied Pharmacology, 216, 168–175.
Khodayar, M. J., Kalantari, H., Khorsandi, L., Rashno, M., & Zeidooni, L. (2018). Betaine protects mice against acetaminophen hepatotoxicity possibly via mitochondrial complex II and glutathione availability. Biomedicine & Pharmacotherapy, 103, 1436–1445.
Dutta, S., Saha, S., Mahalanobish, S., Sadhukhan, P., & Sil, P. C. (2018). Melatonin attenuates arsenic induced nephropathy via the regulation of oxidative stress and inflammatory signaling cascades in mice. Food and Chemical Toxicology, 118, 303–316.
Ogihara, N., & Haley-Vicente, D. (2002). Protein target discovery and characterization-DS modeling and discovery studio streamline target discovery. Genetic Engineering News, 22, 77.
Tissue, E. (1959). Sulfhydryl groups. Archives in Biochemistry and Biophysics, 82, 70–77.
Fossati, P., Prencipe, L., & Berti, G. (1980). Use of 3, 5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clinical Chemistry, 26, 227–231.
Shangari, N., & O’Brien, P. J. (2006). Catalase activity assays. Current Protocols in Toxicology, 27, 1–16.
Srivastava, S. (2020). Arsenic in drinking water and food. Springer.
Yousuf, R., Verma, P. K., Sharma, P., Sood, S., Aït-Kaddour, A., & Bhat, Z. F. (2023). Ameliorative potential of quercetin and catechin against sodium arsenite and mancozeb-induced oxidative renal damage in Wistar rats. Journal of Trace Elements and Minerals, 1, 100079.
Sevim, Ç., Doğan, E., & Comakli, S. (2020). Cardiovascular disease and toxic metals. Current Opinion in Toxicology, 19, 88–92.
Bjørklund, G., Oliinyk, P., Lysiuk, R., Rahaman, M. S., Antonyak, H., Lozynska, I., Lenchyk, L., & Peana, M. (2020). Arsenic intoxication: General aspects and chelating agents. Archives of Toxicology, 94, 1879–1897.
Hauptman, M., & Woolf, A. D. (2022). British anti-lewisite (dimercaprol) (Chapter 34). In A. D. Woolf (Ed.), History of modern clinical toxicology (pp. 243–254). Academic Press.
Bhattacharya, S. (2017). Medicinal plants and natural products in amelioration of arsenic toxicity: A short review. Pharmaceutical Biology, 55, 349–354.
Yu, X., Wang, Z., Shu, Z., Li, Z., Ning, Y., Yun, K., Bai, H., Liu, R., & Liu, W. (2017). Effect and mechanism of Sorbus pohuashanensis (Hante) Hedl flavonoids protect against arsenic trioxide-induced cardiotoxicity. Biomedicine & Pharmacotherapy, 88, 1–10.
Hosseinzadeh, A., Houshmand, G., Goudarzi, M., Sezavar, S. H., Mehrzadi, S., Mansouri, E., & Kalantar, M. (2019). Ameliorative effect of gallic acid on sodium arsenite-induced spleno-, cardio- and hemato-toxicity in rats. Life Sciences, 217, 91–100.
Manna, P., Sinha, M., & Sil, P. C. (2008). Arsenic-induced oxidative myocardial injury: Protective role of arjunolic acid. Archives of Toxicology, 82, 137–149.
Shen, S., Li, X.-F., Cullen, W. R., Weinfeld, M., & Le, X. C. (2013). Arsenic binding to proteins. Chemical Reviews, 113, 7769–7792.
Shi, H., Shi, X., & Liu, K. J. (2004). Oxidative mechanism of arsenic toxicity and carcinogenesis. Molecular and Cellular Biochemistry, 255, 67–78.
Roth, R., & JH, L.J. (2019). Casarett & Doull’s toxicology: The basic science of poisons. McGraw-Hill Education.
Gamble, M. V., Liu, X., Ahsan, H., Pilsner, J. R., Ilievski, V., Slavkovich, V., Parvez, F., Levy, D., Factor-Litvak, P., & Graziano, J. H. (2005). Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environmental Health Perspectives, 113, 1683–1688.
Catena, C., Colussi, G., Url-Michitsch, M., Nait, F., & Sechi, L. A. (2015). Subclinical carotid artery disease and plasma homocysteine levels in patients with hypertension. Journal of the American Society of Hypertension, 9, 167–175.
Ganguly, P., & Alam, S. F. (2015). Role of homocysteine in the development of cardiovascular disease. Nutrition Journal, 14, 1–10.
Kolling, J., Scherer, E. B., Da Cunha, A. A., Da Cunha, M. J., & Wyse, A. T. (2011). Homocysteine induces oxidative–nitrative stress in heart of rats: Prevention by folic acid. Cardiovascular Toxicology, 11, 67–73.
Catena, C., Colussi, G., Nait, F., Capobianco, F., & Sechi, L. A. (2015). Elevated homocysteine levels are associated with the metabolic syndrome and cardiovascular events in hypertensive patients. American Journal of Hypertension, 28, 943–950.
Hoffmann, L., Brauers, G., Gehrmann, T., Häussinger, D., Mayatepek, E., Schliess, F., & Schwahn, B. C. (2013). Osmotic regulation of hepatic betaine metabolism. American Journal of Physiology-Gastrointestinal and Liver Physiology, 304, 835–846.
Navik, U., Sheth, V. G., Kabeer, S. W., & Tikoo, K. (2019). Dietary supplementation of methyl donor l-methionine alters epigenetic modification in type 2 diabetes. Molecular Nutrition & Food Research, 63, 1801401.
Go, E. K., Jung, K. J., Kim, J. M., Lim, H., Lim, H. K., Yu, B. P., & Chung, H. Y. (2007). Betaine modulates age-related NF-κB by thiol-enhancing action. Biological and Pharmaceutical Bulletin, 30, 2244–2249.
Hasanzadeh-Moghadam, M., Khadem-Ansari, M. H., Farjah, G. H., & Rasmi, Y. (2018). Hepatoprotective effects of betaine on liver damages followed by myocardial infarction. Veterinary Research Forum: An International Quarterly Journal, 9, 129–135.
Ganesan, B., Buddhan, S., Jeyakumar, R., & Anandan, R. (2009). Protective effect of betaine on changes in the levels of membrane-bound ATPase activity and mineral status in experimentally induced myocardial infarction in Wistar rats. Biological Trace Element Research, 131, 278–290.
Barchowsky, A., Dudek, E. J., Treadwell, M. D., & Wetterhahn, K. E. (1996). Arsenic induces oxidant stress and NF-kB activation in cultured aortic endothelial cells. Free Radical Biology and Medicine, 21, 783–790.
Balakumar, P., & Kaur, J. (2009). Arsenic exposure and cardiovascular disorders: An overview. Cardiovascular Toxicology, 9, 169–176.
Patel, D., Yadav, P., Singh, S. K., Tanwar, S. S., Sehrawat, A., Khurana, A., Bhatti, J. S., & Navik, U. (2024). Betaine alleviates doxorubicin-induced nephrotoxicity by preventing oxidative insults, inflammation, and fibrosis through the modulation of Nrf2/HO− 1/NLRP3 and TGF-β expression. Journal of Biochemical and Molecular Toxicology, 38, e23559.
Jiang, Y.-P., Yang, J.-M., Ye, R.-J., Liu, N., Zhang, W.-J., Ma, L., Zheng, P., Niu, J.-G., Liu, P., & Yu, J.-Q. (2019). Protective effects of betaine on diabetic induced disruption of the male mice blood-testis barrier by regulating oxidative stress-mediated p38 MAPK pathways. Biomedicine & Pharmacotherapy, 120, 109474.
Go, E. K., Jung, K. J., Kim, J. Y., Yu, B. P., & Chung, H. Y. (2005). Betaine suppresses proinflammatory signaling during aging: The involvement of nuclear factor-κB via nuclear factor-inducing kinase/IκB kinase and mitogen-activated protein kinases. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 60, 1252–1264.
Khodayar, M., Kalantari, H., Khorsandi, L., Rashno, M., & Zeidooni, L. (2020). Upregulation of Nrf2-related cytoprotective genes expression by acetaminophen-induced acute hepatotoxicity in mice and the protective role of betaine. Human & Experimental Toxicology, 39, 948–959.
Urschel, K., & Cicha, I. (2015). TNF-α in the cardiovascular system: From physiology to therapy. International Journal of Interferon, Cytokine and Mediator Research, 7, 9–25.
Gutierrez, S. H., Kuri, M. R., & del Castillo, E. R. (2008). Cardiac role of the transcription factor NF-κB. Cardiovascular & Haematological Disorders-Drug Targets, 8, 153–160.
Berthonneche, C., Sulpice, T., Boucher, F., Gouraud, L., De Leiris, J., O’connor, S., Herbert, J.-M., & Janiak, P. (2004). New insights into the pathological role of TNF-α in early cardiac dysfunction and subsequent heart failure after infarction in rats. American Journal of Physiology-Heart and Circulatory Physiology, 287, 340–350.
Lau, A., Whitman, S. A., Jaramillo, M. C., & Zhang, D. D. (2013). Arsenic-mediated activation of the Nrf2-Keap1 antioxidant pathway. Journal of biochemical and molecular toxicology, 27, 99–105.
Zhang, Y., Wei, Z., Liu, W., Wang, J., He, X., Huang, H., Zhang, J., & Yang, Z. (2017). Melatonin protects against arsenic trioxide-induced liver injury by the upregulation of Nrf2 expression through the activation of PI3K/AKT pathway. Oncotarget, 8, 3773.
Nikravesh, M., Mahdavinia, M., Neisi, N., Khorsandi, L., & Khodayar, M. J. (2023). Citicoline ameliorates arsenic-induced hepatotoxicity and diabetes in mice by overexpression of VAMP2, PPAR-γ, As3MT, and SIRT3. Pesticide Biochemistry and Physiology, 192, 105391.
Acknowledgements
Parts of the graphical abstract were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.
Funding
This study was supported by a student grant from the Student Research Committee (Grant No: 00s86) of AJUMS.
Author information
Authors and Affiliations
Contributions
Data collection, investigation, data analysis, and writing original draft and editing were performed by S.S. Methodology, validation, and consultation were performed by M.S. Methodology and investigation were performed by R.A. Pathological analysis and consultation were performed by L.K. Conceptualization, validation, methodology, writing and editing, and supervision were performed by MJ.K. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors do not have any conflict of interest.
Ethical Approval
Animal experiments working methods and euthanasia were performed based on animal care and use methods approved by the Ethics Committee of AJUMS (Ethics Approval ID: IR.AJUMS.ABHC.REC.1400.115).
Consent for Publication
All authors have consented to the publication of the manuscript.
Additional information
Handling Editor: Lu Cai.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shariati, S., Shirani, M., Azadnasab, R. et al. Betaine Protects Mice from Cardiotoxicity Triggered by Sodium Arsenite Through Antioxidative and Anti-inflammatory Pathways. Cardiovasc Toxicol 24, 539–549 (2024). https://doi.org/10.1007/s12012-024-09864-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-024-09864-3