Abstract
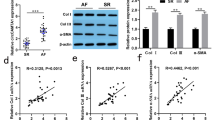
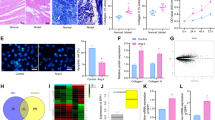
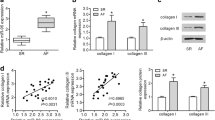
Cartilage oligomeric matrix protein (COMP) regulates transforming growth factor-β (TGF-β) signaling pathway, which has been proved to be associated with skin fibrosis and pulmonary fibrosis. Atrial fibrosis is a major factor of atrial fibrillation (AF). Nevertheless, the interaction between COMP and TGF-β as well as their role in AF remains undefined. The purpose of this study is to clarify the role of COMP in AF and explore its potential mechanism. The hub gene of AF was identified from two datasets using bioinformatics. Furthermore, it was verified by the downregulation of COMP in angiotensin-II (Ang-II)-induced AF in mice. Moreover, the effect on AF was examined using CCK8 assay, ELISA, and western blot. The involvement of TGF-β pathway was further discussed. The expression of COMP was the most significant among all these hub genes. Our experimental results revealed that the protein levels of TGF-β1, phosphorylated Smad2 (P-Smad2), and phosphorylated Smad3 (P-Smad3) were decreased after silencing COMP, which indicated that COMP knockdown could inhibit the activation of TGF-β pathway in AF cells. However, the phenomenon was reversed when the activator SRI was added. COMP acts as a major factor and can improve Ang-II-induced AF via TGF-β signaling pathway. Thus, our research enriches the understanding of the interaction between COMP and TGF-β in AF, and provides reference for the pathogenesis and diagnosis of AF.








Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Yamamoto, C., & Trayanova, N. A. (2022). Atrial fibrillation: Insights from animal models, computational modeling, and clinical studies. eBioMedicine, 85(104310), 26.
Wang, W., Tian, B., Ning, Z., & Li, X. (2022). Research progress of LncRNAs in atrial fibrillation. Molecular Biotechnology, 64(7), 758–772.
Suwa, Y., Miyasaka, Y., Taniguchi, N., Harada, S., Nakai, E., & Shiojima, I. (2022). Atrial fibrillation and stroke: Importance of left atrium as assessed by echocardiography. Journal of Echocardiography, 20(2), 69–76. https://doi.org/10.1007/s12574-021-00561-6
Camm, A. J., Naccarelli, G. V., Mittal, S., Crijns, H., Hohnloser, S. H., Ma, C. S., Natale, A., Turakhia, M. P., & Kirchhof, P. (2022). The increasing role of rhythm control in patients with atrial fibrillation: JACC state-of-the-art review. Journal of the American College of Cardiology, 79(19), 1932–1948.
Wegner, F. K., Plagwitz, L., Doldi, F., Ellermann, C., Willy, K., Wolfes, J., Sandmann, S., Varghese, J., & Eckardt, L. (2022). Machine learning in the detection and management of atrial fibrillation. Clinical Research in Cardiology, 111(9), 1010–1017.
Sehrawat, O., Kashou, A. H., & Noseworthy, P. A. (2022). Artificial intelligence and atrial fibrillation. Journal of Cardiovascular Electrophysiology, 33(8), 1932–1943.
Posey, K. L., Coustry, F., & Hecht, J. T. (2018). Cartilage oligomeric matrix protein: COMPopathies and beyond. Matrix Biology, 71–72, 161–173. https://doi.org/10.1016/j.matbio.2018.02.023
Liang, Y., Fu, Y., Qi, R., Wang, M., Yang, N., He, L., Yu, F., Zhang, J., Yun, C.-H., Wang, X., Liu, J., & Kong, W. (2015). Cartilage oligomeric matrix protein is a natural inhibitor of thrombin. Blood, 126(7), 905–914. https://doi.org/10.1182/blood-2015-01-621292
Nakayama, H., Endo, Y., Aota, S., Sato, M., Fujita, T., & Kikuchi, S. (2003). Novel mutations of the cartilage oligomeric matrix protein (COMP) gene in two Japanese patients with pseudoachondroplasia. Oncology Reports, 10(4), 871–873.
Englund, E., Bartoschek, M., Reitsma, B., Jacobsson, L., Escudero-Esparza, A., Orimo, A., Leandersson, K., Hagerling, C., Aspberg, A., Storm, P., Okroj, M., Mulder, H., Jirstrom, K., Pietras, K., & Blom, A. M. (2016). Cartilage oligomeric matrix protein contributes to the development and metastasis of breast cancer. Oncogene, 35(43), 5585–5596.
Englund, E., Canesin, G., Papadakos, K. S., Vishnu, N., Persson, E., Reitsma, B., Anand, A., Jacobsson, L., Helczynski, L., Mulder, H., Bjartell, A., & Blom, A. M. (2017). Cartilage oligomeric matrix protein promotes prostate cancer progression by enhancing invasion and disrupting intracellular calcium homeostasis. Oncotarget, 8(58), 98298–98311.
Li, Q., Wang, C., Wang, Y., Sun, L., Liu, Z., Wang, L., Song, T., Yao, Y., Liu, Q., & Tu, K. (2018). HSCs-derived COMP drives hepatocellular carcinoma progression by activating MEK/ERK and PI3K/AKT signaling pathways. Journal of Experimental & Clinical Cancer Research, 37(1), 018–0908.
Liu, T. T., Liu, X. S., Zhang, M., Liu, X. N., Zhu, F. X., Zhu, F. M., Ouyang, S. W., Li, S. B., Song, C. L., Sun, H. M., Lu, S., Zhang, Y., Lin, J., Tang, H. M., & Peng, Z. H. (2018). Cartilage oligomeric matrix protein is a prognostic factor and biomarker of colon cancer and promotes cell proliferation by activating the Akt pathway. Journal of Cancer Research and Clinical Oncology, 144(6), 1049–1063.
Fu, Y., Huang, Y., Yang, Z., Chen, Y., Zheng, J., Mao, C., Li, Z., Liu, Z., Yu, B., Li, T., Wang, M., Xu, C., Zhou, Y., Zhao, G., Jia, Y., Guo, W., Jia, X., Zhang, T., Li, L., … Kong, W. (2021). Cartilage oligomeric matrix protein is an endogenous β-arrestin-2-selective allosteric modulator of AT1 receptor counteracting vascular injury. Cell Research, 31(7), 773–790. https://doi.org/10.1038/s41422-020-00464-8
Schulz, J.-N., Plomann, M., Sengle, G., Gullberg, D., Krieg, T., & Eckes, B. (2018). New developments on skin fibrosis - Essential signals emanating from the extracellular matrix for the control of myofibroblasts. Matrix Biology, 68–69, 522–532. https://doi.org/10.1016/j.matbio.2018.01.025
Yu, H., Alruwaili, N., Hu, B., Kelly, M. R., Zhang, B., Sun, D., & Wolin, M. S. (2019). Potential role of cartilage oligomeric matrix protein in the modulation of pulmonary arterial smooth muscle superoxide by hypoxia. American Journal of Physiology. Lung Cellular and Molecular Physiology, 317(5), L569–L577.
Yu, H., Jia, Q., Feng, X., Chen, H., Wang, L., Ni, X., & Kong, W. (2017). Hypoxia decrease expression of cartilage oligomeric matrix protein to promote phenotype switching of pulmonary arterial smooth muscle cells. International Journal of Biochemistry & Cell Biology, 91(Pt A), 37–44.
Wang, H., Yuan, Z., Wang, B., Li, B., Lv, H., He, J., Huang, Y., Cui, Z., Ma, Q., Li, T., Fu, Y., Tan, X., Liu, Y., Wang, S., Wang, C., Kong, W., & Zhu, Y. (2022). COMP (cartilage oligomeric matrix protein), a novel PIEZO1 regulator that controls blood pressure. Hypertension, 79(3), 549–561.
Wang, M., Fu, Y., Gao, C., Jia, Y., Huang, Y., Liu, L., Wang, X., Wang, W., & Kong, W. (2016). Cartilage oligomeric matrix protein prevents vascular aging and vascular smooth muscle cells senescence. Biochemical and Biophysical Research Communications, 478(2), 1006–1013.
Sandstedt, J., Vargmar, K., Bjorkman, K., Ruetschi, U., Bergstrom, G., Hulten, L. M., & Skioldebrand, E. (2021). COMP (cartilage oligomeric matrix protein) neoepitope: A novel biomarker to identify symptomatic carotid stenosis. Arteriosclerosis, Thrombosis, and Vascular Biology, 41(3), 1218–1228.
Yeh, H. W., Lee, S. S., Chang, C. Y., Lang, Y. D., & Jou, Y. S. (2019). A new switch for TGFbeta in cancer. Cancer Research, 79(15), 3797–3805.
Davis, M. D., Suzaki, I., Kawano, S., Komiya, K., Cai, Q., Oh, Y., & Rubin, B. K. (2019). Tissue factor facilitates wound healing in human airway epithelial cells. Chest, 155(3), 534–539.
Seoane, J., & Gomis, R. R. (2017). TGF-beta family signaling in tumor suppression and cancer progression. Cold Spring Harbor Perspectives in Biology, 9(12), a022277.
Peng, D., Fu, M., Wang, M., Wei, Y., & Wei, X. (2022). Targeting TGF-β signal transduction for fibrosis and cancer therapy. Molecular Cancer. https://doi.org/10.1186/s12943-022-01569-x
Ge, C., Zhao, Y., Liang, Y., & He, Y. (2022). Silencing of TLR4 inhibits atrial fibrosis and susceptibility to atrial fibrillation via downregulation of NLRP3-TGF-beta in spontaneously hypertensive rats. Disease Markers, 11, 2466150.
Saljic, A., Grandi, E., & Dobrev, D. (2022). TGF-beta1-induced endothelial–mesenchymal transition: a potential contributor to fibrotic remodeling in atrial fibrillation? Journal of Clinical Investigation. https://doi.org/10.1172/JCI161070
Nattel, S., & Harada, M. (2014). Atrial remodeling and atrial fibrillation: Recent advances and translational perspectives. Journal of the American College of Cardiology, 63(22), 2335–2345.
Woods, C. E., & Olgin, J. (2014). Atrial fibrillation therapy now and in the future: Drugs, biologicals, and ablation. Circulation Research, 114(9), 1532–1546.
Chen, D., Qin, Y., Dai, M., Li, L., Liu, H., Zhou, Y., Qiu, C., Chen, Y., & Jiang, Y. (2020). BGN and COL11A1 regulatory network analysis in colorectal cancer (CRC) reveals that BGN influences CRC cell biological functions and interacts with miR-6828-5p. Cancer Management and Research, 12, 13051–13069. https://doi.org/10.2147/cmar.s277261
Beigel, R., Wunderlich, N. C., Ho, S. Y., Arsanjani, R., & Siegel, R. J. (2014). The left atrial appendage: Anatomy, function, and noninvasive evaluation. JACC: Cardiovascular Imaging, 7(12), 1251–1265.
Dzeshka, M. S., Lip, G. Y., Snezhitskiy, V., & Shantsila, E. (2015). Cardiac fibrosis in patients with atrial fibrillation: Mechanisms and clinical implications. Journal of the American College of Cardiology, 66(8), 943–959.
Ajoolabady, A., Nattel, S., Lip, G. Y. H., & Ren, J. (2022). Inflammasome signaling in atrial fibrillation: JACC state-of-the-art review. Journal of the American College of Cardiology, 79(23), 2349–2366.
Orlov, Y. L., Anashkina, A. A., Klimontov, V. V., & Baranova, A. V. (2021). Medical genetics, genomics and bioinformatics aid in understanding molecular mechanisms of human diseases. International Journal of Molecular Sciences, 22(18), 9962.
Cui, J., & Zhang, J. (2022). Cartilage oligomeric matrix protein, diseases, and therapeutic opportunities. International Journal of Molecular Sciences, 23(16), 9253. https://doi.org/10.3390/ijms23169253
Maly, K., Andres Sastre, E., Farrell, E., Meurer, A., & Zaucke, F. (2021). COMP and TSP-4: Functional roles in articular cartilage and relevance in osteoarthritis. International Journal of Molecular Sciences, 22(5), 2242.
Caron, M. M. J., Janssen, M. P. F., Peeters, L., Haudenschild, D. R., Cremers, A., Surtel, D. A. M., van Rhijn, L. W., Emans, P. J., & Welting, T. J. M. (2020). Aggrecan and COMP improve periosteal chondrogenesis by delaying chondrocyte hypertrophic maturation. Frontiers in Bioengineering and Biotechnology. https://doi.org/10.3389/fbioe.2020.01036
Idriss, N. K., Gamal, R. M., Gaber, M. A., El-Hakeim, E. H., Hammam, N., Ghandour, A. M., Abdelaziz, M. M., & Goma, S. H. (2020). Joint remodeling outcome of serum levels of Dickkopf-1 (DKK1), cartilage oligomeric matrix protein (COMP), and C-telopeptide of type II collagen (CTXII) in rheumatoid arthritis. Central European Journal of Immunology, 45(1), 73–79.
Nishida, Y., Hashimoto, Y., Orita, K., Nishino, K., Kinoshita, T., & Nakamura, H. (2022). Serum cartilage oligomeric matrix protein detects early osteoarthritis in patients with anterior cruciate ligament deficiency. Arthroscopy, 38(3), 873–878.
Zachou, K., Gabeta, S., Shums, Z., Gatselis, N. K., Koukoulis, G. K., Norman, G. L., & Dalekos, G. N. (2017). COMP serum levels: A new non-invasive biomarker of liver fibrosis in patients with chronic viral hepatitis. European Journal of Internal Medicine, 38, 83–88.
Agarwal, P., Schulz, J. N., Blumbach, K., Andreasson, K., Heinegard, D., Paulsson, M., Mauch, C., Eming, S. A., Eckes, B., & Krieg, T. (2013). Enhanced deposition of cartilage oligomeric matrix protein is a common feature in fibrotic skin pathologies. Matrix Biology, 32(6), 325–331.
Inui, S., Shono, F., Nakajima, T., Hosokawa, K., & Itami, S. (2011). Identification and characterization of cartilage oligomeric matrix protein as a novel pathogenic factor in keloids. American Journal of Pathology, 179(4), 1951–1960.
Jansa, V., Klancic, T., Pusic, M., Klein, M., Vrtacnik Bokal, E., Ban Frangez, H., & Rizner, T. L. (2021). Proteomic analysis of peritoneal fluid identified COMP and TGFBI as new candidate biomarkers for endometriosis. Science and Reports, 11(1), 021–00299.
Bartosinska, J., Michalak-Stoma, A., Juszkiewicz-Borowiec, M., Kowal, M., & Chodorowska, G. (2015). The assessment of selected bone and cartilage biomarkers in psoriatic patients from Poland. Mediators of Inflammation, 194535(10), 4.
Denton, N., Pinnick, K. E., & Karpe, F. (2018). Cartilage oligomeric matrix protein is differentially expressed in human subcutaneous adipose tissue and regulates adipogenesis. Molecular Metabolism, 16, 172–179.
Schulz, J., Plomann, M., Sengle, G., Gullberg, D., Krieg, T., & Eckes, B. (2018). New developments on skin fibrosis—Essential signals emanating from the extracellular matrix for the control of myofibroblasts. Matrix Biology: Journal of the International Society for Matrix Biology. https://doi.org/10.1016/j.matbio.2018.01.025
Vuga, L., Milosevic, J., Pandit, K., Ben-Yehudah, A., Chu, Y., Richards, T., Sciurba, J., Myerburg, M., Zhang, Y., Parwani, A., Gibson, K., & Kaminski, N. (2013). Cartilage oligomeric matrix protein in idiopathic pulmonary fibrosis. PLoS ONE, 8(12), e83120. https://doi.org/10.1371/journal.pone.0083120
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
XBC: conceptualization; formal analysis; methodology; writing—original draft; validation; resources; ML: data curation; investigation; software; writing—review & editing; YZ: formal analysis; resources; writing—review & editing; visualization; WY and ZL: methodology; project administration; supervision; writing—review & editing; validation; all authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The author declares that he have no competing interests.
Ethics Approval and Consent to Participate
No human and animal studies were used in this study.
Consent for Publication
Not applicable.
Additional information
Handling Editor: Gerrit Frommeyer.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, X., Li, M., Zhong, Y. et al. COMP Improves Ang-II-Induced Atrial Fibrillation via TGF-β Signaling Pathway. Cardiovasc Toxicol 23, 305–316 (2023). https://doi.org/10.1007/s12012-023-09799-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-023-09799-1