Abstract
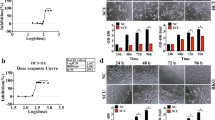
The intake of selenium (Se) in the human body is negatively correlated with the risk of colorectal cancer (CRC), but its mechanism in the occurrence and development of CRC is not clear. This study aimed to evaluate the therapeutic effect of Se on CRC, and explore the anti-tumor effect of Se supplementation on CRC and its molecular mechanism. In this study, we utilized colony formation assay, cell scratch test, Transwell migration, and flow cytometry to assess cell proliferation, migration, and apoptosis. Our findings demonstrate that Se effectively suppresses the growth and proliferation of CRC cell lines HCT116 and SW480 and promoting cellular apoptosis. In vivo experiments demonstrated a significant inhibitory effect of Se on tumor growth. CRC-related datasets were extracted from the Cancer Genome Atlas (TCGA) and the Gene Expression Omnibus (GEO) databases for differential expression analysis of TRIM32 and survival analysis. We found that TRIM32 was highly expressed in tumor tissues of CRC patients and correlated with a poor prognosis. Furthermore, through RNA sequencing analysis, we identified TRIM32 as a gene that was significantly decreased after Se treatment in HCT116 cells. This finding was subsequently validated by Western blot results. Moreover, TRIM32 knockdown combined with Se treatment significantly inhibited cell growth proliferation and migration and further induced apoptosis of colorectal cancer cells. In conclusion, our findings provided evidence that Se inhibited the growth of colorectal cancer cells by down-regulating TRIM32.








Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F (2017) Global patterns and trends in colorectal cancer incidence and mortality. Gut 66(4):683–691. https://doi.org/10.1136/gutjnl-2015-310912
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Siegel RL, Wagle NS, Cercek A, Smith RA (2023) Jemal A (2023) Colorectal cancer statistics. CA Cancer J Clin 73(3):233–254. https://doi.org/10.3322/caac.21772
Nordberg M, Nordberg GF (2016) Trace element research-historical and future aspects. J Trace Elem Med Bio 38:46–52. https://doi.org/10.1016/j.jtemb.2016.04.006
Rayman MP (2000) The importance of selenium to human health. The Lancet 356(9225):233–241. https://doi.org/10.1016/S0140-6736(00)02490-9
Kielczykowska M, Kocot J, Pazdzior M, Musik I (2018) Selenium - a fascinating antioxidant of protective properties. Adv Clin Exp Med 27(2):245–255. https://doi.org/10.17219/acem/67222
Kieliszek M (2019) Selenium(-) fascinating microelement, properties and sources in food. Molecules 24(7). https://doi.org/10.3390/molecules24071298
Vinceti M, Filippini T, Del GC, Dennert G, Zwahlen M, Brinkman M, Zeegers MP, Horneber M, D’Amico R, Crespi CM (2018) Selenium for preventing cancer. Cochrane Database Syst Rev 1:D5195. https://doi.org/10.1002/14651858.CD005195.pub4
Fedirko V, Jenab M, Meplan C, Jones JS, Zhu W, Schomburg L, Siddiq A, Hybsier S, Overvad K, Tjonneland A, Omichessan H, Perduca V, Boutron-Ruault MC, Kuhn T, Katzke V, Aleksandrova K, Trichopoulou A, Karakatsani A, Kotanidou A, Tumino R, Panico S, Masala G, Agnoli C, Naccarati A, Bueno-De-Mesquita B, Vermeulen R, Weiderpass E, Skeie G, Nost TH, Lujan-Barroso L, Quiros JR, Huerta JM, Rodriguez-Barranco M, Barricarte A, Gylling B, Harlid S, Bradbury KE, Wareham N, Khaw KT, Gunter M, Murphy N, Freisling H, Tsilidis K, Aune D, Riboli E, Hesketh JE, Hughes DJ (2019) Association of selenoprotein and selenium pathway genotypes with risk of colorectal cancer and interaction with selenium status. Nutrients 11(4) https://doi.org/10.3390/nu11040935
Kudva AK, Shay AE, Prabhu KS (2015) Selenium and inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 309(2):G71–G77. https://doi.org/10.1152/ajpgi.00379.2014
Ye R, Huang J, Wang Z, Chen Y, Dong Y (2021) Trace element selenium effectively alleviates intestinal diseases. Int J Mol Sci 22(21):11708. https://doi.org/10.3390/ijms222111708
Watanabe M, Hatakeyama S (2017) TRIM proteins and diseases. J Biochem 161(2):135–144. https://doi.org/10.1093/jb/mvw087
Hatakeyama S (2017) TRIM family proteins: roles in autophagy, immunity, and carcinogenesis. Trends Biochem Sci 42(4):297–311. https://doi.org/10.1016/j.tibs.2017.01.002
Eberhardt W, Haeussler K, Nasrullah U, Pfeilschifter J (2020) Multifaceted roles of TRIM proteins in colorectal carcinoma. Int J Mol Sci 21(20). https://doi.org/10.3390/ijms21207532
Marzano F, Caratozzolo MF, Pesole G, Sbisa E, Tullo A (2021) TRIM proteins in colorectal cancer: TRIM8 as a promising therapeutic target in chemo resistance. Biomedicines 9(3) https://doi.org/10.3390/biomedicines9030241
Liu M, Zhang X, Cai J, Li Y, Luo Q, Wu H, Yang Z, Wang L, Chen D (2018) Downregulation of TRIM58 expression is associated with a poor patient outcome and enhances colorectal cancer cell invasion. Oncol Rep 40(3):1251–1260. https://doi.org/10.3892/or.2018.6525
Lazzari E, Meroni G (2016) TRIM32 ubiquitin E3 ligase, one enzyme for several pathologies: from muscular dystrophy to tumours. Int J Biochem Cell Biol 79:469–477. https://doi.org/10.1016/j.biocel.2016.07.023
Cambiaghi V, Giuliani V, Lombardi S, Marinelli C, Toffalorio F, Pelicci PG (2012) TRIM proteins in cancer. Adv Exp Med Biol 770:77–91. https://doi.org/10.1007/978-1-4614-5398-7_6
Locke M, Tinsley CL, Benson MA, Blake DJ (2009) TRIM32 is an E3 ubiquitin ligase for dysbindin. Hum Mol Genet 18(13):2344–2358. https://doi.org/10.1093/hmg/ddp167
Ito M, Migita K, Matsumoto S, Wakatsuki K, Tanaka T, Kunishige T, Nakade H, Nakatani M, Nakajima Y (2017) Overexpression of E3 ubiquitin ligase tripartite motif 32 correlates with a poor prognosis in patients with gastric cancer. Oncol Lett 13(5):3131–3138. https://doi.org/10.3892/ol.2017.5806
Wang C, Xu J, Fu H, Zhang Y, Zhang X, Yang D, Zhu Z, Wei Z, Hu Z, Yan R, Cai Q (2018) TRIM32 promotes cell proliferation and invasion by activating β-catenin signalling in gastric cancer. J Cell Mol Med 22(10):5020–5028. https://doi.org/10.1111/jcmm.13784
Zhao T, Jin F, Li J, Xu Y, Dong H, Liu Q, Xing P, Zhu G, Xu H, Yin S, Miao Z (2018) TRIM32 promotes proliferation and confers chemoresistance to breast cancer cells through activation of the NF-κB pathway. J Cancer 9(8):1349–1356. https://doi.org/10.7150/jca.22390
Yin H, Li Z, Chen J, Hu X (2019) Expression and the potential functions of TRIM32 in lung cancer tumorigenesis. J Cell Biochem 120(4):5232–5243. https://doi.org/10.1002/jcb.27798
Su X, Wang B, Wang Y, Wang B (2020) Inhibition of TRIM32 induced by miR-519d increases the sensitivity of colorectal cancer cells to cisplatin. Onco Targets Ther 13:277–289. https://doi.org/10.2147/OTT.S235940
Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W (2022) Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 135(5):584–590. https://doi.org/10.1097/CM9.0000000000002108
Dariya B, Aliya S, Merchant N, Alam A, Nagaraju GP (2020) Colorectal cancer biology, diagnosis, and therapeutic approaches. Crit Rev Oncog 25(2):71–94. https://doi.org/10.1615/CritRevOncog.2020035067
Wrobel JK, Power R, Toborek M (2016) Biological activity of selenium: revisited. IUBMB Life 68(2):97–105. https://doi.org/10.1002/iub.1466
Schrauzer GN (2009) Selenium and selenium-antagonistic elements in nutritional cancer prevention. Crit Rev Biotechnol 29(1):10–17. https://doi.org/10.1080/07388550802658048
Keskin H, Wang SM, Etemadi A, Fan JH, Dawsey SM, Abnet CC, Qiao YL, Taylor PR (2021) Colorectal cancer in the Linxian China Nutrition Intervention Trial: risk factors and intervention results. PLoS ONE 16(9):e255322. https://doi.org/10.1371/journal.pone.0255322
Hesketh J, Meplan C (2011) Transcriptomics and functional genetic polymorphisms as biomarkers of micronutrient function: focus on selenium as an exemplar. Proc Nutr Soc 1–9. https://doi.org/10.1017/S0029665111000115
Meplan C, Hesketh J (2012) The influence of selenium and selenoprotein gene variants on colorectal cancer risk. Mutagenesis 27(2):177–186. https://doi.org/10.1093/mutage/ger058
Wang H, Liu H, Zhou M, Shi H, Shen M (2020) Correlations between 13 trace elements and circulating tumor cells in patients with colorectal cancer in Guangzhou China. Biol Trace Elem Res 198(1):58–67. https://doi.org/10.1007/s12011-020-02061-7
Meplan C, Johnson IT, Polley AC, Cockell S, Bradburn DM, Commane DM, Arasaradnam RP, Mulholland F, Zupanic A, Mathers JC, Hesketh J (2016) Transcriptomics and proteomics show that selenium affects inflammation, cytoskeleton, and cancer pathways in human rectal biopsies. Faseb J 30(8):2812–2825. https://doi.org/10.1096/fj.201600251R
Pohl NM, Tong C, Fang W, Bi X, Li T, Yang W (2009) Transcriptional regulation and biological functions of selenium-binding protein 1 in colorectal cancer in vitro and in nude mouse xenografts. PLoS ONE 4(11):e7774. https://doi.org/10.1371/journal.pone.0007774
Zhang X, Hong R, Bei L, Yang J, Zhao X, Hu Z, Chen L, Meng H, Zhang Q, Niu G, Yue Y, Ke C (2022) Selenium binding protein 1 inhibits tumor angiogenesis in colorectal cancers by blocking the delta-like ligand 4/Notch1 signaling pathway. Transl Oncol 18:101365. https://doi.org/10.1016/j.tranon.2022.101365
Jaworska AM, Wlodarczyk NA, Mackiewicz A, Czerwinska P (2020) The role of TRIM family proteins in the regulation of cancer stem cell self-renewal. Stem Cells 38(2):165–173. https://doi.org/10.1002/stem.3109
Han Y, Tan Y, Zhao Y, Zhang Y, He X, Yu L, Jiang H, Lu H, Tian H (2020) TRIM23 overexpression is a poor prognostic factor and contributes to carcinogenesis in colorectal cancer. J Cell Mol Med 24(10):5491–5500. https://doi.org/10.1111/jcmm.15203
Horn EJ, Albor A, Liu Y, El-Hizawi S, Vanderbeek GE, Babcock M, Bowden GT, Hennings H, Lozano G, Weinberg WC, Kulesz-Martin M (2004) RING protein Trim32 associated with skin carcinogenesis has anti-apoptotic and E3-ubiquitin ligase properties. Carcinogenesis 25(2):157–167. https://doi.org/10.1093/carcin/bgh003
Zhang B, Yan YY, Gu YQ, Teng F, Lin X, Zhou XL, Che JX, Dong XW, Zhou LX, Lin NM (2022) Inhibition of TRIM32 by ibr-7 treatment sensitizes pancreatic cancer cells to gemcitabine via mTOR/p70S6K pathway. J Cell Mol Med 26(2):515–526. https://doi.org/10.1111/jcmm.17109
Wang J, Fang Y, Liu T (2020) TRIM32 promotes the growth of gastric cancer cells through enhancing AKT activity and glucose transportation. Biomed Res Int 2020:4027627. https://doi.org/10.1155/2020/4027627
Liu J, Zhang C, Wang XL, Ly P, Belyi V, Xu-Monette ZY, Young KH, Hu W, Feng Z (2014) E3 ubiquitin ligase TRIM32 negatively regulates tumor suppressor p53 to promote tumorigenesis. Cell Death Differ 21(11):1792–1804. https://doi.org/10.1038/cdd.2014.121
Kano S, Miyajima N, Fukuda S, Hatakeyama S (2008) Tripartite motif protein 32 facilitates cell growth and migration via degradation of Abl-interactor 2. Cancer Res 68(14):5572–5580. https://doi.org/10.1158/0008-5472.CAN-07-6231
Prajapati P, Gohel D, Shinde A, Roy M, Singh K, Singh R (2020) TRIM32 regulates mitochondrial mediated ROS levels and sensitizes the oxidative stress induced cell death. Cell Signal 76:109777. https://doi.org/10.1016/j.cellsig.2020.109777
Soukupova K, Rudolf E (2019) Suppression of proliferation and activation of cell death by sodium selenite involves mitochondria and lysosomes in chemoresistant bladder cancer cells. J Trace Elem Med Biol 52:58–67. https://doi.org/10.1016/j.jtemb.2018.11.009
Jeong JY, Wang Y, Sytkowski AJ (2009) Human selenium binding protein-1 (hSP56) interacts with VDU1 in a selenium-dependent manner. Biochem Biophys Res Commun 379(2):583–588. https://doi.org/10.1016/j.bbrc.2008.12.110
Fontelles CC, Ong TP (2017) Selenium and breast cancer risk: focus on cellular and molecular mechanisms. Adv Cancer Res 136:173–192. https://doi.org/10.1016/bs.acr.2017.08.001
Saeed AM, Mohamed HR, Ahmed KM (2019) The pro-oxidant, apoptotic and anti-angiogenic effects of selenium supplementation on colorectal tumors induced by 1,2-dimethylhydrazine in BALB/C mice. Rep Biochem Mol Biol 8(3):216–226
Acknowledgements
We thank the associate editor and the reviewers for their useful feedback that improved this paper.
Funding
This study was financially supported by National Natural Science Foundation of China (no. 82073414) and Basic and Applied Basic Research Foundation of Guangdong Province (no. 2021A1515010715).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Xiaohua Cai: Conceptualization, Methodology, Investigation, Writing – original draft, Writing – review & editing, Project administration. Yintong Su: Investigation, Methodology, Formal analysis. Jiayu Ning: Validation, Formal analysis. Xingxing Fan: Resources. Mei Shen: Conceptualization, Supervision, Writing – review & editing, Funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The study was reviewed and approved by the Medical Ethics Committee of Southern Medical University (no. 2021–0024) and the Experimental Animal Ethics Committee of Southern Medical University (L2019074).
Consent to Participate
All study participants signed informed consent.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, X., Su, Y., Ning, J. et al. Research on the Effect and Mechanism of Selenium on Colorectal Cancer Through TRIM32. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04206-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04206-4