Abstract
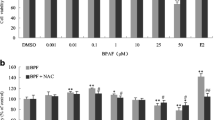
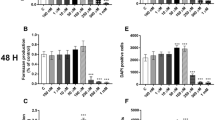
Although humans are frequently exposed to multiple pollutants simultaneously, research on their harmful effects on health has typically focused on studying each pollutant individually. Inorganic arsenic (As) and benzo[a]pyrene (BaP) are well-known pollutants with carcinogenic potential, but their co-exposure effects on breast cancer cell progression remain incompletely understood. This study aimed to assess the combined impact of BaP and As on the viability and migration of MDA-MB-231 cells. The results indicated that even at low levels, both inorganic As (0.01 μM, 0.1 μM, and 1 μM) and BaP (1 μM, 2.5 μM), individually or in combination, enhanced the viability and migration of the cells. However, the cell cycle analysis revealed no significant differences between the control group and the cells exposed to BaP and As. Specifically, exposure to BaP alone or in combination with As (As 0.01 μM + BaP 1 μM) for 24 h led to a significant increase in vimentin gene expression. Interestingly, short-term exposure to As not only did not induce EMT but also modulated the effects of BaP on vimentin gene expression. However, there were no observable changes in the expression of E-cadherin mRNA. Consequently, additional research is required to evaluate the prolonged effects of co-exposure to As and BaP on the initiation of EMT and the progression of breast cancer.




Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Feng Y, Spezia M, Huang S, Yuan C, Zeng Z, Zhang L et al (2018) Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis 5(2):77–106
Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F (2021) Cancer statistics for the year 2020: an overview. Int J Cancer 149(4):778–789
Assi HA, Khoury KE, Dbouk H, Khalil LE, Mouhieddine TH, El Saghir NS (2013) Epidemiology and prognosis of breast cancer in young women. J Thorac Dis 5(Suppl 1):2–8
Momenimovahed Z, Salehiniya H (2019) Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer (Dove Med Press) 11:151–164
Russo J, Russo IH (2006) The role of estrogen in the initiation of breast cancer. J Steroid Biochem Mol Biol 102(1–5):89–96
Sun YS, Zhao Z, Yang ZN, Xu F, Lu HJ, Zhu ZY et al (2017) Risk factors and preventions of breast cancer. Int J Biol Sci 13(11):1387–1397
Watkins EJ (2019) Overview of breast cancer. JAAPA 32(10):13–17
Winters S, Martin C, Murphy D, Shokar NK (2017) Breast cancer epidemiology, prevention, and screening. Prog Mol Biol Transl Sci 151:1–32
Korsh J, Shen A, Aliano K, Davenport T (2015) polycyclic aromatic hydrocarbons and breast cancer: a review of the literature. Breast Care (Basel) 10(5):316–318
Boffetta P (2006) Human cancer from environmental pollutants: the epidemiological evidence. Mutat Res 608(2):157–162
Chaffer CL, Weinberg RA (2011) A perspective on cancer cell metastasis. Science 331(6024):1559–1564
Bergers G, Fendt S-M (2021) The metabolism of cancer cells during metastasis. Nat Rev Cancer 21(3):162–180
Ganesh K, Massagué J (2021) Targeting metastatic cancer. Nat Med 27(1):34–44
Geiger TR, Peeper DS (2009) Metastasis mechanisms. Biochim Biophys Acta 1796(2):293–308
Georgakopoulos-Soares I, Chartoumpekis DV, Kyriazopoulou V, Zaravinos A (2020) EMT factors and metabolic pathways in cancer. Front Oncol 10:499
Babaei G, Aziz SG-G, Jaghi NZZ (2021) EMT, cancer stem cells and autophagy; the three main axes of metastasis. Biomed Pharmacother 133:110909
Brabletz T, Kalluri R, Nieto MA, Weinberg RA (2018) EMT in cancer. Nat Rev Cancer 18(2):128–134
Landrigan PJ, Fuller R, Fisher S, Suk WA, Sly P, Chiles TC, Bose-O’Reilly S (2019) Pollution and children’s health. Sci Total Environ 650:2389–2394
Bopp SK, Barouki R, Brack W, Dalla Costa S, Dorne J-LC, Drakvik PE et al (2018) Current EU research activities on combined exposure to multiple chemicals. Environ Int 120:544–562
Lagunas-Rangel FA, Linnea-Niemi JV, Kudłak B, Williams MJ, Jönsson J, Schiöth HB (2022) Role of the synergistic interactions of environmental pollutants in the development of cancer. GeoHealth 6(4):e2021GH000552
You M, Song Y, Chen J, Liu Y, Chen W, Cen Y et al (2023) (2023) Combined exposure to benzo (a) pyrene and dibutyl phthalate aggravates pro-inflammatory macrophage polarization in spleen via pyroptosis involving cathepsin B. Sci Total Environ 881:163460
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2004) Some drinking-water disinfectants and contaminants, including arsenic. IARC Monogr Eval Carcinog Risks Hum 84:1–477
Pullella K, Kotsopoulos J (2020) Arsenic exposure and breast cancer risk: a re-evaluation of the literature. Nutrients 12(11):1–17
Abernathy CO, Liu YP, Longfellow D, Aposhian HV, Beck B, Fowler B et al (1999) Arsenic: health effects, mechanisms of actions, and research issues. Environ Health Perspect 107(7):593–597
Martínez-Castillo M, García-Montalvo EA, Arellano-Mendoza MG, Sánchez-Peña LdC, Soria Jasso LE, Izquierdo-Vega JA et al (2021) Arsenic exposure and non-carcinogenic health effects. Hum Exp Toxicol 40(12_suppl):S826–S50
Marciniak W, Matoušek T, Domchek S, Paradiso A, Patruno M, Irmejs A et al (2021) Blood arsenic levels as a marker of breast cancer risk among BRCA1 carriers. Cancers 13(13):3345
Palma-Lara I, Martínez-Castillo M, Quintana-Pérez J, Arellano-Mendoza M, Tamay-Cach F, Valenzuela-Limón O et al (2020) Arsenic exposure: a public health problem leading to several cancers. Regul Toxicol Pharmacol 110:104539
Marciniak W, Derkacz R, Muszyńska M, Baszuk P, Gronwald J, Huzarski T et al (2020) Blood arsenic levels and the risk of familial breast cancer in Poland. Int J Cancer 146(10):2721–2727
López-Carrillo L, Gamboa-Loira B, Gandolfi AJ, Cebrián ME (2020) Inorganic arsenic methylation capacity and breast cancer by immunohistochemical subtypes in northern Mexican women. Environ Res 184:109361
Amadou A, Praud D, Coudon T, Deygas F, Grassot L, Faure E et al (2021) Risk of breast cancer associated with long-term exposure to benzo [a] pyrene (BaP) air pollution: evidence from the French E3N cohort study. Environ Int 149:106399
Gao M, Zheng A, Chen L, Dang F, Liu X, Gao J (2022) Benzo (a) pyrene affects proliferation with reference to metabolic genes and ROS/HIF-1α/HO-1 signaling in A549 and MCF-7 cancer cells. Drug Chem Toxicol 45(2):741–749
Guo J, Xu Y, Ji W, Song L, Dai C, Zhan L (2015) Effects of exposure to benzo [a] pyrene on metastasis of breast cancer are mediated through ROS-ERK-MMP9 axis signaling. Toxicol Lett 234(3):201–210
Kasala ER, Bodduluru LN, Barua CC, Sriram CS, Gogoi R (2015) Benzo(a)pyrene induced lung cancer: role of dietary phytochemicals in chemoprevention. Pharmacol Rep 67(5):996–1009
Bjørklund G, Tippairote T, Rahaman MS, Aaseth J (2020) Developmental toxicity of arsenic: a drift from the classical dose–response relationship. Arch Toxicol 94(1):67–75
Wang Z (2021) Mechanisms of the synergistic lung tumorigenic effect of arsenic and benzo (a) pyrene combined-exposure. Semin Cancer Biol 76:156–162
Koual M, Tomkiewicz C, Cano-Sancho G, Antignac J-P, Bats A-S, Coumoul X (2020) Environmental chemicals, breast cancer progression and drug resistance. Environ Health 19:1–25
Lewandowska AM, Rudzki M, Rudzki S, Lewandowski T, Laskowska B (2018) Environmental risk factors for cancer-review paper. Ann Agric Environ Med 26(1):1–7
Reyes-Vázquez L, Hernández AJA, Calderón-Aranda ES (2020) Role of aromatase activation on sodium arsenite-induced proliferation, migration, and invasion of MDA-MB-231 and MDA-MB-453 breast cancer cell lines. Toxicol 437:152440
Yang P, Xie J, Li Y, Lin H-P, Fenske W, Clementino M et al (2020) Deubiquitinase USP7-mediated MCL-1 up-regulation enhances Arsenic and Benzo (a) pyrene co-exposure-induced Cancer Stem Cell-like property and Tumorigenesis. Theranostics 10(20):9050
Shim Y, Song JM (2015) Spectral overlap-free quantum dot-based determination of benzo [a] pyrene-induced cancer stem cells by concurrent monitoring of CD44, CD24 and aldehyde dehydrogenase 1. Chem Commun 51(11):2118–2121
Clément F, Xu X, Donini CF, Clément A, Omarjee S, Delay E et al (2017) Long-term exposure to bisphenol A or benzo (a) pyrene alters the fate of human mammary epithelial stem cells in response to BMP2 and BMP4, by pre-activating BMP signaling. Cell Death & Differ 24(1):155–166
Shadboorestan A, Tarfiei GA, Montazeri H, Sepand MR, Zangooei M, Khedri A et al (2019) Invasion and migration of MDA-MB-231 cells are inhibited by block of AhR and NFAT: role of AhR/NFAT1/β4 integrin signaling. J Appl Toxicol 39(2):375–384
Foo NP, Ko CL, Chu CY, Wang CY, So EC (2020) Huang BM (2020) Arsenic compounds activate the MAPK and caspase pathways to induce apoptosis in OEC-M1 gingival epidermal carcinoma. Oncol Rep 44(6):2701–2714
Mu YF, Chen YH, Chang MM, Chen YC, Huang BM (2019) Arsenic compounds induce apoptosis through caspase pathway activation in MA-10 Leydig tumor cells. Oncol Lett 18(1):944–954
Jiang R, Li Y, Xu Y, Zhou Y, Pang Y, Shen L et al (2013) EMT and CSC-like properties mediated by the IKKβ/IκBα/RelA signal pathway via the transcriptional regulator, Snail, are involved in the arsenite-induced neoplastic transformation of human keratinocytes. Archiv toxicol 87:991–1000
Rea M, Kimmerer G, Mittendorf S, Xiong X, Green M, Chandler D, et al (2024) A dynamic model of inorganic arsenic-induced carcinogenesis reveals an epigenetic mechanism for epithelial–mesenchymal plasticity. Environ Pollut 347:1–10
Chen X, Peng H, Xiao J, Guan A, Xie B, He B, Chen Q (2017) Benzo(a)pyrene enhances the EMT-associated migration of lung adenocarcinoma A549 cells by upregulating Twist1. Oncol Rep 38(4):2141–2147
Vergara-Gerónimo CA, León-Del-Rio A, Rodríguez-Dorantes M, Camacho-Carranza R, Ostrosky-Wegman P, Salazar AM (2023) Arsenic reduces the GATA3 expression associated with an increase in proliferation and migration of mammary epithelial cell line MCF-10A. Toxicol Appl Pharmacol 472:116573
Danes JM, de Abreu ALP, Kerketta R, Huang Y, Palma FR, Gantner BN et al (2020) Inorganic arsenic promotes luminal to basal transition and metastasis of breast cancer. FASEB 34(12):16034–16048
Shadboorestan A, Koual M, Dairou J, Coumoul X (2023) The role of the kynurenine/AhR pathway in diseases related to metabolism and cancer. Int J Tryptophan Res 16:11786469231185102
Wang Z, Fu Y, Seno A, Bi Z, Pawar AS, Ji H, et al (2023) Tumor suppressive activity of AHR in environmental arsenic-induced carcinogenesis. Toxicol Appl Pharmacol 480:1–7
Thompson ED, Burwinkel KE, Chava AK, Notch EG, Mayer GD (2010) Activity of phase I and phase II enzymes of the benzo [a] pyrene transformation pathway in zebrafish (Danio rerio) following waterborne exposure to arsenite. Comp Biochem Physiol C Toxicol Pharmacol 152(3):371–378
Vakharia DD, Liu N, Pause R, Fasco M, Bessette E, Zhang Q-Y, Kaminsky LS (2001) Polycyclic aromatic hydrocarbon/metal mixtures: effect on PAH induction of CYP1A1 in human HEPG2 cells. Drug Metab Dispos 29(7):999–1006
Wang Z, Yang P, Xie J, Lin HP, Kumagai K, Harkema J, Yang C (2020) Arsenic and benzo[a]pyrene co-exposure acts synergistically in inducing cancer stem cell-like property and tumorigenesis by epigenetically down-regulating SOCS3 expression. Environ Int 137:105560
Xie J, Yang P, Lin HP, Li Y, Clementino M, Fenske W et al (2020) Integrin α4 up-regulation activates the hedgehog pathway to promote arsenic and benzo[α]pyrene co-exposure-induced cancer stem cell-like property and tumorigenesis. Cancer Lett 493:143–155
Li Y, He M, Chen B, Hu B (2019) Inhibition of arsenite methylation induces synergistic genotoxicity of arsenite and benzo (a) pyrene diol epoxide in SCC-7 cells. Metallomics 11(1):176–182
Shen S, Lee J, Cullen WR, Le XC, Weinfeld M (2009) Arsenite and its mono-and dimethylated trivalent metabolites enhance the formation of benzo [a] pyrene diol epoxide− DNA adducts in xeroderma pigmentosum complementation group A cells. Chem Res Toxicol 22(2):382–390
Acknowledgements
The authors of this manuscript wish to express their appreciation to Tarbiat Modares University of Medical Sciences, Tehran, Iran.
Funding
This work was supported by the Tarbiat Modares University grant to accomplish Ahmad Safari Maleki's M.Sc. thesis [Grant number 87424].
Author information
Authors and Affiliations
Contributions
Amir Shadboorestan and Mohammad Hossein Ghahremani contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Ahmad Safari Maleki and Amir Shadboorestan. The manuscript has been written, revised, and approved by all authors involved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maleki, A.S., Ghahremani, M.H. & Shadboorestan, A. Arsenic and Benzo[a]pyrene Co-exposure Effects on MDA-MB-231 Cell Viability and Migration. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04170-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04170-z