Abstract
Honey bees are commonly exposed to a broad spectrum of xenobiotics, including heavy metals. Heavy metal toxicity is of concern in the context of global pollinator declines, especially since honey bees seem to be particularly susceptible to xenobiotics in general. Here we summarize current knowledge on the interplay between cadmium, one of the most toxic and mobile elements in the environment, and honey bees, the primary managed pollinator species worldwide. Overall, cadmium pollution has been shown to be ubiquitous, affecting industrial, urban and rural areas alike. Uptake of this heavy metal by plants serves as the primary route of exposure for bees (through pollen and nectar). Reported cadmium toxicity consists of lethal and sublethal effects (reduced development and growth) in both adult and larval stages, as well as various molecular responses related to detoxification and cellular antioxidant defence systems. Other effects of cadmium in honey bees include the disruption of synaptic signalling, calcium metabolism and muscle function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The honey bee (Apis mellifera L., 1758) is an economically significant domestic insect valued for numerous products including honey, beeswax, pollen, propolis and royal jelly [1]. Additionally, the ecosystem services provided by these animals in terms of pollination of wild plants and agricultural crops are arguably even more important [2, 3]. Bees contribute through these services to the preservation of plant biodiversity and consequently all associated organisms higher up in the food chain. Likewise, pollinators play a key role in agricultural sustainability and food security in a modern climate [4,5,6]. However, a decrease in economic gain, stability of pollination and bee populations has been described across the Western world [4, 7]. In the last decades, wild and domestic pollinators have been experiencing severe declines, raising considerable concern within the scientific community, as well as in the rural sector (agriculture and beekeeping) [6, 8,9,10,11,12,13,14,15]. Although the multitude of interacting factors behind these losses are far from being fully understood, bee declines have been coupled with the increasing effects of pests and diseases (e.g. the ectoparasitic mite Varroa destructor), pesticide use, climate change, feed shortage (melliferous plants),and the intensification of agricultural practices causing habitat and forage biodiversity loss [9, 11, 16, 17]. In this complex background, environmental contamination, including heavy metal pollution, is believed to have significant consequences for bee health, contributing to these declines [3, 15, 18, 19].
Since the late nineteenth century, increasing anthropological activities related to industrial, agricultural and urban outputs have caused a steep increase in environmental heavy metal burdens around the globe. This, in combination with the fact that heavy metals do not decompose, has led these contaminants to commonly be found in the atmosphere, soil and water, as well as in numerous organisms after entering biological cycles [18, 20,21,22]. In general, honey bees are exposed to mineral elements through food and water, and this is no different for heavy metals [15]. Accumulation of heavy metals has previously been shown in plants, including in nectar and pollen, the main feed recourses gathered by honey bees [22,23,24,25,26,27,28].Other routes of contamination for bees include the inhalation of contaminated airborne particles and adhesion to their hairy exterior from soil, plants and atmospheric deposition [29,30,31].
Though metals like copper (Cu), zinc (Zn) and selenium (Se) are crucial trace elements for insect metabolism, other elements like cadmium (Cd), lead (Pb), mercury (Hg) and arsenic (As) have no known physiological function within the insect body [15, 31]. The latter are believed to be toxic even at low concentrations. Overall, toxic heavy metals interfere with biological processes through interaction with macromolecules and/or replacing/affecting the function of essential elements in other ways [15, 18, 32, 33]. In insects, heavy metals have been reported to cause cellular structural and genetic damage among others, potentially disrupting cell functionality and causing apoptosis and mutations. Furthermore, negative effects on insect survival, development, growth and reproduction have been pointed out [22].
While the specific adverse effects of various heavy metals on pollinators are still largely unknown [19], recent efforts have been undertaken to define the effects of toxic metals on honey bee development and survival, including from a physiological and biochemical point of view [18, 23, 34,35,36,37,38,39,40,41]. Furthermore, heavy metals in bees and their products, as well as the interplay with environmental contamination, have received considerable attention [5, 42,43,44,45,46,47,48,49,50,51,52,53,54]. Lastly, the potential role of honey bees and their products as bioindicators for toxic metal pollution has been highlighted [14, 15, 29, 30, 39, 45, 55,56,57,58,59,60,61,62,63,64,65,66].
In light of this, this review outlines the interrelation between honey bees and Cd, one of the most toxic and mobile elements in the environment [35, 67], based on the most relevant scientific literature. This review means to centralize the current knowledge in a comprehensive manner in the hope of instigating further research and management efforts in mitigating heavy-metal-related pollinator declines. A summarizing figure of the main Cd emission sources, contamination routes for bees and the effects of this heavy metal on honey bees is shown in Fig. 1.
Cadmium (Cd)
Cadmium is a highly toxic metal harmful to humans’, animals’ and plants’ health [68, 69]. Cadmium is naturally present in soil, the lithosphere and sedimentary rock [70] and is often found together with zinc, lead and copper ores [68]. Natural dispersion of Cd results from airborne soil particles, forest fires, volcanic activity, soil erosion and the abrasion of rocks [67, 69, 70]. Annual natural emission is reported at 1300–41,000 tons [67] and the global soil concentration of this heavy metal is estimated at 0.07–1.1 mg kg−1 in natural ecosystems [71]. The concentration of this toxicant in different matrices, as well as based on geographical location and/or soil type, can be found in the current scientific literature [22, 67, 68, 71].
Elevated presence of Cd in the environment is mainly accredited to anthropogenic sources linked to a wide range of human activities [69, 72]. Specifically, mining, smelting and refining of various metals, fossil fuel combustion, cement production, the use of phosphate fertilizers and municipal and sewage sludge incineration are common sources of Cd pollution. Additionally, the use of Cd in electronic devices, batteries and solar panels has led to the leaching of this toxicant from landfill sites into the environment. Lastly, industrial use of Cd as a corrosive reagent, stabilizer in PVC products and in colour pigments generates a continued source of Cd contamination [35, 67, 69,70,71,72,73].
Due to its specific chemical characteristics, Cd and its compounds are easily dispersed through water, polluting rivers and natural bodies of water located in the vicinity of emission sources [69, 70]. Airborne Cd can be dispersed over short and long distances and is deposited ubiquitously, including with rainwater [67, 69, 70]. Soil contamination results from the deposition of airborne particles, dispersion through water and the large variety of natural and anthropogenic Cd sources discussed above [69, 72]. As a result, Cd pollution is widespread around mining, urban, agricultural and industrial areas [69, 74] and can even affect seemingly “unpolluted” environments [15, 66]. Finally, given the bio-accumulative properties of Cd in both plants and animals (with an approximate half-life of 25–30 years), this pollutant is commonly found in a wide range of organisms including mushrooms, rice, wheat, vegetables, crustaceans, molluscs and honey bees [15, 68,69,70, 75,76,77].
Cd Exposure of Bees
Bees are exposed to Cd mostly through contaminated plants [15, 22]. Pollen and nectar, the main feed resources collected by honey bees, have been shown to be contaminated with Cd [39, 42, 61, 78,79,80] and bees not to avoid contaminated feed recourses as they most likely cannot discriminate between metal-contaminated and non-contaminated plants [19, 74]. Furthermore, a dose-dependent relationship between the Cd content of bees and the concentration of this contaminant in their feed has been found [35]. Besides this, bees are exposed to Cd through inhalation and deposition of airborne particles [22, 35, 40, 45, 46, 50, 60]. Recent research comparing bee whole-body Cd concentrations with that in their haemolymph revealed significant differences (lower haemolymph concentrations) which could be accredited to the accumulation of Cd-contaminated dust particles on the bee’s hairy exterior surface [15]. External Cd in turn could be ingested by bees during grooming [22]. Alternatively, Sadowska et al. [49] report the contribution of externally adhered Cd to the whole-body content of this metal in bees to be negligible.
As Cd pollution pressure differs according to spatial and temporal variations, honey bee exposure trends are complex and highly dependent on their immediate environment [15, 22, 42, 43, 45, 46, 49, 50, 60, 61]. First of all, variations in Cd pollution pressure for bees might result from the availability of different plant resources for foraging [20,21,22, 78]. Indeed, Cd contents will vary substantially between plant species as well as between cultivars and genotypes of the same species [68]. This being said, limited information is currently available on the metal concentrations in the pollen and especially nectar of different plants [22].
Next, Cd contamination will vary based on the general location of apiaries, likely related to the influence of both natural and anthropogenic emission sources. In this regard, significant Cd burdens have been found in honey bees in industrial, urban, agricultural and rural areas around the globe [15, 39, 42, 43, 45, 46, 50, 53, 60, 62, 64, 68]. A recent survey analysing the Cd content of bees from various areas in Serbia revealed bees residing in the vicinity of industrial zones to be exposed to significantly higher Cd pollution as compared to those from urban or rural areas [15], as previously reported [20, 22, 48, 50]. In Sardinia, bees located near a mining area were more affected by this contaminant [44]. In Italy, Poland and Turkey, Cd contamination was greater in urban than in rural areas [29, 49, 53, 64, 66]. Substantial Cd contamination in bees from agricultural zones [15, 22, 42, 43, 45, 60, 62] and seemingly unpolluted rural areas [15, 62, 64, 66] has been reported as well which, in certain cases, was even higher as compared to industrial zones [15, 20, 60, 64].
Significant temporal variations in Cd contamination of bees have been highlighted within the same study area [15, 42,43,44, 60]. Such variations are hypothesised to be the result of seasonal changes in dominant Cd dispersion pathways. For example, airborne Cd might represent a major contamination route during winter as climatic conditions promote atmospheric particle deposition [15]. Additionally, increased airborne Cd could be related to the elevated combustion of fossil fuels at those times [15]. Supporting this hypothesis, toxicant monitoring of deposited airborne particles by [15] revealed substantially higher Cd contents in winter, especially near industrial areas. Alternatively, seasonal variations in Cd exposure might be correlated to the state of activity of honey bees in general [15]. Overall, non-essential element concentrations in bees have been shown to be higher during spring and summer [15, 20, 44, 60] when bees are most active and thus have maximal contact with toxicants.
Lastly, significant differences in Cd pressure on a smaller time scale have been pointed out as well [45, 64]. van der Steen et al. [45] found significant variations in the Cd contents of bees from the same location over a 3-month period and argued that in highly populated and developed countries, fluctuations in anthropogenic Cd emission serve as the main factor influencing Cd pollution pressure for honey bees.
Effects of Cd on Honey Bees
Cadmium-Related Mortality and Altered Development
Current knowledge on the impacts of Cd on honey bee survival is still limited [23, 35, 81]. Nevertheless, lethal and sublethal effects of Cd exposure on larval and adult honey bees have been shown, even at ecologically relevant concentrations [35]. Specifically, reduced development and growth and increased mortality have been pointed out [23, 35, 81].
Cronn [81] first studied the lethal effects of Cd on adult bees and determined this toxicant to be moderate to highly toxic for honey bees. In a series of experimental trials, dietary supplementation of Cd to young nurse bees revealed a significant increase in mortality occurring as early as 24 h after initiation of the experiment. Median lethal doses (LD50) for oral intake of two Cd salts over various lengths of exposure (for CdCl2, 3.51 µg Cd/bee for 48 h, 2.80 µg Cd/bee for 96 h; for CdSO4, 2.34 µg Cd/bee for 48 h, 1.44 µg Cd/bee for 96 h) were reported [81]. Analogous results were published by Di et al. [35] where Cd consumption by honey bee foragers (dissolved in 50% sucrose solution) led to increased mortality both over time and with increasing dose. Furthermore, mortality was shown to increase more rapidly with increasing Cd treatment and a lethal concentration (LC50) of Cd of 78 mg L−1 was reported [35]. Other research efforts focussing on the physiological responses of honey bees to Cd revealed no lethal effects on adult individuals for supplementation ranging between 0.001 and 0.1 mg L−1 over a period of 2–10 days [18, 28, 31, 36].
Di et al. [35] reported the results of acute and chronic Cd toxicity tests on honey bee larvae through dosing of an artificial diet (53% W/W commercial freshly frozen royal jelly, 6% glucose, 6% fructose, 1% yeast extract, and 34% ultrapure water), showing substantial variation as compared to foragers. In this regard, authors revealed sublethal effects on larval development in addition to a dose-dependent increase in mortality [35]. Negative effects on larval growth and development were characterised by significantly lower pupal weight for animals treated with 3.16 mg L−1 of Cd and reduced growth rates starting from 1.05 mg L−1 onwards. A significant difference in mortality was seen starting from day 4 after initiation of the experiment. For the highest Cd-doses (9.47–28.41 mg L−1), mortality of 100% was shown after less than 1 week and some treatment groups never even reached the pupal stage. These results highlight the particularly high toxicity of Cd for honey bee larvae with a LC50 of 0.275 mg L−1 [35]. These values correspond to realistic Cd burdens for honey bees, suggesting larval survival to potentially be affected by Cd pollution under field conditions [35].
Besides these effects at the individual level, it should be considered that increased adult and brood mortality as well as altered development will likely have negative impacts on whole colony health [25, 35, 37]. However, colony-level impact studies of heavy metals, including Cd, are lacking. To the best of our knowledge, only one research investigated the effects of Cd on whole bee hives and revealed oral supplementation of environmentally realistic concentrations (through sugar syrup and pollen patties) for 60 days to have an effect on larval honey bee stages in particular [28]. Specifically, Cd treatment reduced pupal survival significantly, indicating high brood mortality to be a real threat to colonies commonly exposed to Cd. Although no difference in total worker weight was recorded, authors argue prolonged Cd exposure (exceeding 60 days) could reduce overall worker populations over time [28]. Earlier research reporting fewer adult bees and reduced productivity in hives located near heavy metal–contaminated industrial areas supports this hypothesis [82].
Molecular Response to Cd Toxicity
Studies investigating the molecular responses of honey bees to Cd are relatively more copious. Overall, efforts have been made to identify the effects of this pollutant on detoxification and cellular antioxidant defence systems in particular [18, 28, 34, 36,37,38,39, 82, 83]. These effects are discussed together in the next paragraphs as both systems share various key aspects. Other molecular effects of Cd in honey bees include the disruption of synaptic signalling, calcium metabolism and muscle function.
Metallothionein
Metallothioneins (MTs) are a superfamily of metal-binding proteins present in all eukaryotes which play crucial roles in metal homeostasis and detoxification. These molecules are central for increasing metal tolerance, reducing toxic metal burdens, and are known to protect organisms against the toxic effects of metals, especially Cd [84, 85]. In fact, the initial description of MTs was as Cd‐binding proteins in the kidneys of horses [86].
Experimental research [81] first uncovered Cd-binding proteins identified as MTs in honey bees. This author reported a close to linear rise in MTs with increasing Cd supplementation and time, and honey bee mortality seemed to increase slower as compared to MT accumulation, indicating a protective function [81]. Production of such proteins was also reported in bees from Cd-contaminated areas, suggesting environmental Cd exposure to induce MT [34, 81].
The Apis mellifera metallothionein gene(AmMT) was only recently identified, characterised and sequenced and was found to code for a single protein [38]. Purać et al. [38] went on to test the Cd detoxification capacity of this protein through overexpression of recombinant AmMT in Escherichia coli revealing this to cause increased metal tolerance. Further laboratory and field experiments with honey bee workers showed a dose‐dependent relationship between Cd contamination and AmMT expression [38]. This was expected as Purać et al. [38] identified a metal-induced transcription factor promotion region flanking the honey bee MT gene, as previously found in other animals, including insects [87]. An increase in MT expression resulting from Cd exposure has also been reported in Musca domestica, Folsomia candida and Orchesella cincta [88,89,90]. Research efforts [38, 81] have provided the cornerstone for our understanding of metal homeostasis and regulation in honey bees and the potential role of MTs in metal detoxification and tolerance of Cd.
Besides their role in metal homeostasis and detoxification, MTs play an important function in cellular antioxidant defence systems. This is achieved firstly through the binding of metals preventing the production of free radicals and secondly by the direct scavenging of reactive oxygen species [84, 91]. Moreover, molecular signalling related to oxidative stress is known to induce MT expression as well [92, 93] and has been identified in honey bees [38]. Hence, Cd-induced MT expression in honey bees could (at least partly) be due to oxidative stress response.
Oxidative Stress
Heavy metals and their ions are known to cause oxidative stress by generating an increase in free radicals and highly reactive oxygen species [32]. Under normal circumstances, the cellular antioxidant defence system (superoxide dismutase family; SOD, catalase; CAT, among others) neutralizes these threats, but in the presence of excess amounts of toxicants (like heavy metals), defences are depleted, leading to oxidative damage [94]. Specifically, Cd is known to impede cellular antioxidant defence systems through the inhibition, depletion and/or replacement of essential elements, indirectly leading to oxidative stress [95]. The effects of Cd exposure can consequently be quantified by measuring the levels and activity of various components of the antioxidant defence mechanism, as well as the amount of oxidative damage in cells.
The effects of environmentally realistic Cd concentrations on the cellular antioxidant defence system of honey bees were investigated [18]. Authors revealed the feeding of Cd-contaminated sucrose (0.01–0.1 mg L−1) to adult worker bees to cause upregulation of Cat, Sod1 and Sod2 gene expressions, genes coding for the enzymes CAT and SOD respectively [96]. A linear dose-dependent increase in Cat and a non-linear dose-dependent increase in Sod gene expression was found [18], in correspondence with previous research in other invertebrates [97,98,99]. Upregulation of these genes shows an increased need to eliminate oxidizing radicals and thus oral Cd treatment to cause a heightened risk of oxidative stress in honey bees [18]. Additionally, an increased expression of antioxidant enzyme genes (including Cat and Sod) was found in bees from areas contaminated with heavy metals, further suggesting this to be a protective adaptation to heavy metal–mediated oxidative stress, even though contamination by Cd specifically was not investigated in these studies [5, 37].Lastly, no concrete evidence of increased oxidative damage was found by [18] after 48 h, though authors argue prolonged exposure to Cd might generate clearer results.
Follow-up research by [37] explored the effects of oral exposure of honey bees to Cd on another group of important protective enzymes. There, authors revealed, in accordance with comparable experiments in other arthropods [100, 101], a dose-dependent increase in gene expression of three classes of glutathione S-transferase (GST: Delta;Gstd1, Sigma;Gsts1, microsomal;Gstmic1). These are multifunctional enzymes that play crucial functions in the management of oxidative stress, including glutathione peroxidise activity and the processing of endogenous reactive intermediates and oxidative metabolites [102, 103]. Analogous to their previous findings, a more profound effect on the gene level was observed in comparison with the enzyme activity (which was not altered for GST in response to Cd), indicating gene expression to be a more accurate reflection of the acute toxic effects of Cd [18, 37]. Again, as experimental conditions only lasted for 48 h, prolonged exposure to Cd could lead to distinct results.
Lastly, α-tocopherol and carotenoid levels (well-known dietary molecules in support of cell endogenous antioxidant defence systems) do not seem to be affected by Cd supplementation in honey bees [28, 36].
Genetic Background
It is worth mentioning that honey bees have fewer genes coding for enzymes of cellular detoxification and antioxidative systems as compared to other insects [104]. For example, Apis mellifera holds approximately one-third of the genes for GST present in some other insect species [105]. Likewise, only one MT gene was found in honey bees [38], while at least two such genes have been identified in many invertebrates, with one coding for a specific Cd-binding MT [85]. Moreover, MT gene duplication in early research with fruit flies was found to double their MT production capacity leading to a heightened tolerance to metals, including Cd [81]. Consequently, Apis mellifera could be especially prone to effects from intoxication by heavy metals and xenobiotics in general [34, 37, 83, 105]. Alternatively, it has been speculated that other forms of defence against toxicants might be in place in honey bee colonies where caste structure, behaviour and the dilution of toxicants play a role [23, 105].
Neurotransmission
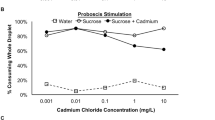
Previously mentioned experimental research [83] investigated the effects of Cd on acetylcholinesterase (ACE) activity in honey bees as well and showed a reduced activity of this hydrolysing enzyme in response to 0.001 and 0.01 mg L−1of Cd. ACE is an enzyme responsible for the metabolization of the neurotransmitter acetylcholine (in both vertebrates and invertebrates)following its release into cholinergic-type chemical synapses, including those found in neuromuscular junctions. The breakdown of acetylcholine leads to the termination of synaptic transmission and the inactivation of ACE effectively causes disruption of neurotransmission following synaptic acetylcholine accumulation and receptor hyperstimulation [106, 107]. In fact, commonly applied pesticides (e.g. organophosphorus and carbamate pesticides) specifically target and inhibit this enzyme, causing paralysis and consequentially death in insects [106, 108]. Cadmium is believed to inhibit ACE by directly binding to the enzyme, compromising its functionality either through loss of activity or deterioration [83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109].
Even though Cd is a known inhibitor of ACE, the effects of various metals on this enzyme have been shown to be species-specific [83, 109,110,111,112,113,114]. Regarding Cd and honey bees specifically, results presented by Nikolić et al. [81] indicate a clear inhibitory function on whole-body enzyme activity. While the consequences of ACE inhibition on honey bee locomotion and mortality were not explored in the mentioned research, authors do make an interesting point suggesting the effects of toxicants with a similar mode of action could be exacerbated due to additive toxicity caused by Cd. Consequently, environmental exposure of honey bees to sublethal concentrations of pesticides with synergistic effects could prove lethal in combination with Cd contamination [81].
Effects of Cd on honey bee locomotion might result from the disturbance of alternative molecular mechanisms. For instance, Cd could affect ACE activity by interfering with calcium metabolism [113, 115, 116]. Moreover, Cd has been found to block voltage-dependent Ca channels in honey bee skeletal muscle fibres, resulting in modified action potential [117]. These transmembrane channels regulating the passage of positive calcium ions are key components of membrane depolarisation and inhibition of this mechanism can therefore result in impaired neuromuscular transmission, muscle contraction and locomotion [118, 119]. This could be especially true as calcium flow is of particular importance within the mechanisms of depolarisation and muscle contraction in insects (including honey bees) compared to sodium [117, 118]. Besides, as was discussed for ACE inhibitors, an analogous additive effect of Cd on ion channel–targeting pesticides can be hypothesised [118]. One recent finding by Li and co-workers [120] linked the exposure to Cd and the depressed olfactory ability of foragers. This effect could potentially impact the localization ability of feeding source and limit the ecological role of pollination by bees. The effect of carry-over into honey [121] plays as a gatherer of interest within the scientific community not only of the effects on bee physiology and patho-physiology, playing as environmental sentinels of pollutions, but also for the role in the potential load of toxic elements into honey, as food for human consumption.
Conclusion
The complex nature of the interplay between honey bees and environmental Cd pollution is real. While significant efforts have been put toward defining spatial patterns of contamination, research regarding Cd contents of feed (pollen and especially nectar), being the chief mode of exposure for bees, is still lacking. Realistic Cd burdens appear to represent a serious threat to honey bees in terms of development and survival and larval stages to be especially susceptible to the toxic effects of Cd. Additionally, the genetic background pointed out in this review suggests honey bees to be particularly sensitive to xenobiotics in general, including heavy metals. However, there is a need to further evaluate the effects of Cd on both adult and larval stages under field conditions. A deepening of our understanding of metal homeostasis and the molecular responses of honey bees to Cd is needed as well. Lastly, significant synergetic adverse effects between Cd and other stressors (e.g. other heavy metals and pesticides) have been accounted. In the context of understanding global pollinator and honey bee declines, investigation into heavy metal toxicity deserves continuous attention.
Data availability
Not applicable.
References
Becsi B, Formayer H, Brodschneider R (2021) A biophysical approach to assess weather impacts on honey bee colony winter mortality. Royal Soc Open Sci 8(9):210618
van Engelsdorp D, Meixner MD (2010) A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J Invertebr Pathol 103:S80–S95
Di Noi A, Casini S, Campani T, Cai G, Caliani I (2021) Review on sublethal effects of environmental contaminants in honey bees (Apis mellifera), knowledge gaps and future perspectives. Int J Environ Res Public Health 18(4):1863
Leonhardt SD, Gallai N, Garibaldi LA, Kuhlmann M, Klein AM (2013) Economic gain, stability of pollination and bee diversity decrease from southern to northern Europe. Basic Appl Ecol 14(6):461–471
Gizaw G, Kim Y, Moon K, Choi JB, Kim YH, Park JK (2020) Effect of environmental heavy metals on the expression of detoxification-related genes in honey bee Apis mellifera. Apidologie 51:664–674
López-Uribe MM, Ricigliano VA, Simone-Finstrom M (2020) Defining pollinator health: a holistic approach based on ecological, genetic, and physiological factors. Ann Rev Anim Biosci 8:269–294
Garibaldi LA, Aizen MA, Klein AM, Cunningham SA, Harder LD (2011) Global growth and stability of agricultural yield decrease with pollinator dependence. Proc Natl Acad Sci 108(14):5909–5914
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25(6):345–353
Goulson D, Nicholls E, Botías C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347(6229):1255957
Jacques A, Laurent M, Ribiere-Chabert M, Saussac M, Bougeard S, Hendrikx P, Chauzat MP (2016) Statistical analysis on the EPILOBEE dataset: explanatory variables related to honeybee colony mortality in EU during a 2 year survey. EFSA Supporting Publications 13(4):883E
Kulhanek K, Steinhauer N, Rennich K, Caron DM, Sagili RR, Pettis JS, vanEngelsdorp D (2017) A national survey of managed honey bee 2015–2016 annual colony losses in the USA. J Apic Res 56(4):328–340
Brodschneider R, Gray A, Adjlane N, Ballis A, Brusbardis V, Charrière JD, Danihlík J (2018) Multi-country loss rates of honey bee colonies during winter 2016/2017 from the COLOSS survey. J Apic Res 57(3):452–457
Oberreiter H, Brodschneider R (2020) Austrian COLOSS survey of honey bee colony winter losses 2018/19 and analysis of hive management practices. Diversity 12(3):99
Costa A, Veca M, Barberis M, Cicerinegri L, Tangorra FM (2021) Predicting atmospheric cadmium and lead using honeybees as atmospheric heavy metals pollution indicators Results of a monitoring survey in Northern Italy. Ital J Anim Sci 20(1):850–858
Ilijević K, Vujanović D, Orčić S, Purać J, Kojić D, Zarić N, Čelić TV (2021) Anthropogenic influence on seasonal and spatial variation in bioelements and non-essential elements in honeybees and their hemolymph. Comp Biochem Physiol C Toxicol Pharmacol 239:108852
Stanimirović Z, Glavinić U, Ristanić M, Aleksić N, Jovanović NM, Vejnović B, Stevanović J (2019) Looking for the causes of and solutions to the issue of honey bee colony losses. Acta Vet-Beogr 69(1):1–31
Hristov P, Shumkova R, Palova N, Neov B (2021) Honey bee colony losses: Why are honey bees disappearing? Sociobiology 68(1):e5851–e5851
Nikolić TV, Kojić D, Orčić S, Batinić D, Vukašinović E, Blagojević DP, Purać J (2016) The impact of sublethal concentrations of Cu, Pb and Cd on honey bee redox status, superoxide dismutase and catalase in laboratory conditions. Chemosphere 164:98–105
Burden CM, Morgan MO, Hladun KR, Amdam GV, Trumble JJ, Smith BH (2019) Acute sublethal exposure to toxic heavy metals alters honey bee (Apis mellifera) feeding behavior. Sci Rep 9(1):4253
Roman A (2010) Levels of copper, selenium, lead, and cadmium in forager bees. Polish J Environ Stud 19(3)
Al Naggar YA, Naiem ESA, Seif AI, Mona MH (2013) Honey bees and their products as a bio-indicator of environmental pollution with heavy metals. Mellifera 13:1–20
Di N, Zhang K, Hladun KR, Rust M, Chen YF, Zhu ZY, Trumble JT (2020) Joint effects of cadmium and copper on Apis mellifera forgers and larvae. Comp Biochem Physiol C Toxicol Pharmacol 237:108839
Hladun KR, Di N, Liu TX, Trumble JT (2016) Metal contaminant accumulation in the hive: consequences for whole-colony health and brood production in the honey bee (Apis mellifera L.). Environ Toxicol Chem 35(2):322–329
Hladun KR, Parker DR, Trumble JT (2011) Selenium accumulation in the floral tissues of two Brassicaceae species and its impact on floral traits and plant performance. Environ Exp Bot 74:90–97
Hladun KR, Parker DR, Trumble JT (2015) Cadmium, copper, and lead accumulation and bioconcentration in the vegetative and reproductive organs of Raphanus sativus: implications for plant performance and pollination. J Chem Ecol 41:386–395
Quinn CF, Prins CN, Freeman JL, Gross AM, Hantzis LJ, Reynolds RJ, Pilon-Smits EA (2011) Selenium accumulation in flowers and its effects on pollination. New Phytol 192(3):727–737
Meindl GA, Ashman TL (2014) Nickel accumulation by Streptanthus polygaloides (Brassicaceae) reduces floral visitation rate. J Chem Ecol 40:128–135
Gauthier M, Aras P, Jumarie C, Boily M (2016) Low dietary levels of Al, Pb and Cd may affect the non-enzymatic antioxidant capacity in caged honey bees (Apis mellifera). Chemosphere 144:848–854
Perugini M, Manera M, Grotta L, Abete MC, Tarasco R, Amorena M (2011) Heavy metal (Hg, Cr, Cd, and Pb) contamination in urban areas and wildlife reserves: honeybees as bioindicators. Biol Trace Elem Res 140:170–176
van der Steen JJ, Martel AC, Hendrickx P (2015) The fraction haemolymph vitellogenin of a honey bee colony, derived from a pooled haemolymph sample, a colony vitality parameter. J Apic Res 54(1):55–58
Dabour K, Al Naggar Y, Masry S, Naiem E, Giesy JP (2019) Cellular alterations in midgut cells of honey bee workers (Apis millefera L.) exposed to sublethal concentrations of CdO or PbO nanoparticles or their binary mixture. Sci Total Environ 651:1356–1367
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment Molecular, clinical and environmental toxicology: volume 3: environmental toxicology 133–164
Knoll S, Pinna W, Varcasia A, Scala A, Cappai MG (2020) The honey bee (Apis mellifera L., 1758) and the seasonal adaptation of productions Highlights on summer to winter transition and back to summer metabolic activity. A review. Livest Sci 235:104011
Badiou-Bénéteau A, Benneveau A, Géret F, Delatte H, Becker N, Brunet JL, Belzunces LP (2013) Honeybee biomarkers as promising tools to monitor environmental quality. Environ Int 60:31–41
Di N, Hladun KR, Zhang K, Liu TX, Trumble JT (2016) Laboratory bioassays on the impact of cadmium, copper and lead on the development and survival of honeybee (Apis mellifera L.) larvae and foragers. Chemosphere 152:530–538
Jumarie C, Aras P, Boily M (2017) Mixtures of herbicides and metals affect the redox system of honey bees. Chemosphere 168:163–170
Nikolić TV, Kojić D, Orčić S, Vukašinović EL, Blagojević DP, Purać J (2019) Laboratory bioassays on the response of honey bee (Apis mellifera L.) glutathione S-transferase and acetylcholinesterase to the oral exposure to copper, cadmium, and lead. Environ Sci Pollut Res 26:6890–6897
Purać J, Nikolić TV, Kojić D, Ćelić AS, Plavša JJ, Blagojević DP, Petri ET (2019) Identification of a metallothionein gene in honey bee Apis mellifera and its expression profile in response to Cd. Mol Ecol 28(4):731–745
Al Naggar Y, Dabour K, Masry S, Sadek A, Naiem E, Giesy JP (2020) Sublethal effects of chronic exposure to CdO or PbO nanoparticles or their binary mixture on the honey bee (Apis millefera L.). Environ Sci Pollut Res 27:19004–19015
Monchanin C, Drujont E, Devaud JM, Lihoreau M, Barron AB (2021) Metal pollutants have additive negative effects on honey bee cognition. J Exp Biol 224(12):241869
Li Z, Qiu Y, Li J, Wan K, Nie H, Su S (2022) Chronic cadmium exposure induces impaired olfactory learning and altered brain gene expression in honey bees (Apis mellifera). Insects 13(11):988
Roman A (2005) The influence of environment on accumulation of toxic elements in honey bees’ body. ISAH 2:423–426
Roman A (2007) Content of some trace elements in fresh honeybee pollen. Pol J Food Nutr Sci 57(4C):475–478
Satta A, Verdinelli M, Ruiu L, Buffa F, Salis S, Sassu A, Floris I (2012) Combination of beehive matrices analysis and ant biodiversity to study heavy metal pollution impact in a post-mining area (Sardinia, Italy). Environ Sci Pollut Res 19:3977–3988
van der Steen JJ, de Kraker J, Grotenhuis T (2012) Spatial and temporal variation of metal concentrations in adult honeybees (Apis mellifera L.). Environ Monit Assess 184:4119–4126
Van der Steen JJM, Cornelissen B, Blacquière T, Pijnenburg JEML, Severijnen M (2016) Think regionally, act locally: metals in honeybee workers in the Netherlands (surveillance study 2008). Environ Monit Assess 188:1–9
Formicki G, Greń A, Stawarz R, Zyśk B, Gał A (2013) Metal content in honey, propolis, wax, and bee pollen and implications for metal pollution monitoring. Pol J Environ Stud 22(1)
Giglio A, Ammendola A, Battistella S, Naccarato A, Pallavicini A, Simeon E, Giulianini PG (2017) Apis mellifera ligustica, Spinola 1806 as bioindicator for detecting environmental contamination: a preliminary study of heavy metal pollution in Trieste, Italy. Environ Sci Pollut Res 24:659–665
Sadowska M, Gogolewska H, Pawelec N, Sentkowska A, Krasnodębska-Ostręga B (2019) Comparison of the contents of selected elements and pesticides in honey bees with regard to their habitat. Environ Sci Pollut Res 26:371–380
Goretti E, Pallottini M, Rossi R, La Porta G, Gardi T, Goga BC, Cappelletti D (2020) Heavy metal bioaccumulation in honey bee matrix, an indicator to assess the contamination level in terrestrial environments. Environ Pollut 256:113388
Smith KE, Weis D (2020) Evaluating spatiotemporal resolution of trace element concentrations and Pb isotopic compositions of honeybees and hive products as biomonitors for urban metal distribution. GeoHealth 4(7):e2020GH000264
Mahé C, Jumarie C, Boily M (2021) The countryside or the city: Which environment is better for the honeybee? Environ Res 195:110784
Bayir H, Aygun A (2022) Heavy metal in honey bees, honey, and pollen produced in rural and urban areas of Konya province in Turkey. Environ Sci Pollut Res 29(49):74569–74578
Scivicco M, Squillante J, Velotto S, Esposito F, Cirillo T, Severino L (2022) Dietary exposure to heavy metals through polyfloral honey from Campania region (Italy). J Food Compos Anal 114:104748
Porrini C, Ghini S, Girotti S (2002) Use of honey bees as bioindicators of environmental pollution in Italy. In: Porrini C, Ghini S, Girotti S, Sabatini AG (eds) In Honey bees. CRC Press, pp 200–261
Porrini C, Sabatini AG, Girotti S, Ghini S, Medrzycki P, Grillenzoni F, Celli G (2003) Honey bees and bee products as monitors of the environmental contamination. Apiacta 38(1):63–70
Celli G, Maccagnani B (2003) Honey bees as bioindicators of environmental pollution. Bull Insectol 56(1):137–139
Zugravu CA, Parvu M, Patrascu D, Stoian A (2009) Correlations between lead and cadmium pollution of honey and environmental heavy metal presence in two Romanian counties. Bull UASVM Agric 66:230–233
Ruschioni S, Riolo P, Minuz RL, Stefano M, Cannella M, Porrini C, Isidoro N (2013) Biomonitoring with honeybees of heavy metals and pesticides in nature reserves of the Marche Region (Italy). Biol Trace Elem Res 154:226–233
Gutiérrez M, Molero R, Gaju M, van der Steen J, Porrini C, Ruiz JA (2015) Assessment of heavy metal pollution in Córdoba (Spain) by biomonitoring foraging honeybee. Environ Monit Assess 187:1–15
Gutiérrez M, Molero R, Gaju M, van der Steen J, Porrini C, Ruiz JA (2020) Assessing heavy metal pollution by biomonitoring honeybee nectar in Córdoba (Spain). Environ Sci Pollut Res 27:10436–10448
Ćirić J, Spirić D, Baltić T, Lazić IB, Trbović D, Parunović N, Đorđević V (2021) Honey bees and their products as indicators of environmental element deposition. Biol Trace Elem Res 199(6):2312–2319
Cunningham MM, Tran L, McKee CG, Polo RO, Newman T, Lansing L, Guarna MM (2022) Honey bees as biomonitors of environmental contaminants, pathogens, and climate change. Ecol Indic 134:108457
Di Fiore C, Nuzzo A, Torino V, De Cristofaro A, Notardonato I, Passarella S, Avino P (2022) Honeybees as bioindicators of heavy metal pollution in urban and rural areas in the South of Italy. Atmosphere 13(4):624
Zarić NM, Brodschneider R, Goessler W (2022) Honey bees as biomonitors–variability in the elemental composition of individual bees. Environ Res 204:112237
Goretti E, Pallottini M, La Porta G, Elia AC, Gardi T, Petroselli C, Cappelletti D (2023) Bioaccumulation of trace elements along the body longitudinal axis in honey bees. Appl Sci 13(12):6918
Kubier A, Wilkin RT, Pichler T (2019) Cadmium in soils and groundwater: a review. Appl Geochem 108:104388
Shahid M, Dumat C, Khalid S, Niazi NK, Antunes PM (2017) Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. Rev Environ Contam Toxicol 241:73–137
Genchi G, Sinicropi MS, Lauria G, Carocci A, Catalano A (2020) The effects of cadmium toxicity. Int J Environ Res Public Health 17(11):3782
Mahmood Q, Asif M, Shaheen S, Hayat MT, Ali S (2019) Cadmium contamination in water and soil. In: Cadmium toxicity and tolerance in plants. Academic Press, pp 141–161
Kumar A, Subrahmanyam G, Mondal R, Cabral-Pinto MMS, Shabnam AA, Jigyasu DK, Yu ZG (2021) Bio-remediation approaches for alleviation of cadmium contamination in natural resources. Chemosphere 268:128855
Benavides MP, Gallego SM, Tomaro ML (2005) Cadmium toxicity in plants. Braz J Plant Physiol 17:21–34
Yuan Z, Luo T, Liu X, Hua H, Zhuang Y, Zhang X, Ren J (2019) Tracing anthropogenic cadmium emissions: from sources to pollution. Sci Total Environ 676:87–96
McLaughlin R, Sivakoff FS, Gardiner MM (2018) Effects of cadmium soil contamination on pollination services (Doctoral dissertation, The Ohio State University)
Ahmad K, Ashfaq A, Khan ZI, Bashir H, Sohail M, Mehmood N, Dogan Y (2018) Metal accumulation in Raphanus sativus and Brassica rapa: an assessment of potential health risk for inhabitants in Punjab, Pakistan. Environ Sci Pollut Res 25:16676–16685
Khan ZI, Ugulu I, Sahira S, Ahmad K, Ashfaq A, Mehmood N, Dogan Y (2018) Determination of toxic metals in fruits of Abelmoschus esculentus grown in contaminated soils with different irrigation sources by spectroscopic method. Int J Environ Res 12:503–511
Ugulu I, Khan ZI, Sahira S, Ahmad K, Mehmood N, Dogan Y (2022) Determination of heavy metal accumulation in wastewater irrigated pumpkin (Cucurbita maxima Duch.) by spectroscopic method. Arab J Geosci 15(14):1238
Bogdanov S (2006) Contaminants of bee products. Apidologie 37(1):1–18
Morgano MA, Teixeira Martins MC, Rabonato LC, Milani RF, Yotsuyanagi K, Rodriguez-Amaya DB (2010) Inorganic contaminants in bee pollen from southeastern Brazil. J Agric Food Chem 58(11):6876–6883
Henson TM, Cory W, Rutter MT (2013) Extensive variation in cadmium tolerance and accumulation among populations of Chamaecrista fasciculata. PLoS One 8(5):e63200
Cronn RC (1991) Determination of cadmium toxicity and the relationship between dose and metallothionein levels in the honey bee, Apis mellifera.Graduate Student Theses, Dissertations, & Professional Papers 2032. https://scholarworks.umt.edu/etd/2032
Bromenshenk JJ, Gudatis JL, Carlson SR, Thomas JM, Simmons MA (1991) Population dynamics of honey bee nucleus colonies exposed to industrial pollutants. Apidologie 22(4):359–369
Nikolić TV, Purać J, Orčić S, Kojić D, Vujanović D, Stanimirović Z, Blagojević DP (2015) Environmental effects on superoxide dismutase and catalase activity and expression in honey bee. Arch Insect Biochem Physiol 90(4):181–194
Coyle P, Philcox JC, Carey LC, Rofe AM (2002) Metallothionein: the multipurpose protein. Cell Mol Life Sci 59:627–647
Luo M, Finet C, Cong H, Wei HY, Chung H (2020) The evolution of insect metallothioneins. Proc R Soc B 287(1937):20202189
Margoshes M, Vallee BL (1957) A cadmium protein from equine kidney cortex. J Am Chem Soc 79(17):4813–4814
Selvaraj A, Balamurugan K, Yepiskoposyan H, Zhou H, Egli D, Georgiev O, Schaffner W (2005) Metal-responsive transcription factor (MTF-1) handles both extremes, copper load and copper starvation, by activating different genes. Genes Dev 19(8):891–896
Sterenborg I, Roelofs D (2003) Field-selected cadmium tolerance in the springtail Orchesella cincta is correlated with increased metallothionein mRNA expression. Insect Biochem Mol Biol 33(7):741–747
Nakamori T, Fujimori A, Kinoshita K, Ban-nai T, Kubota Y, Yoshida S (2010) mRNA expression of a cadmium-responsive gene is a sensitive biomarker of cadmium exposure in the soil collembolan Folsomia candida. Environ Pollut 158(5):1689–1695
Tang T, Huang DW, Zhang D, Wu YJ, Murphy RW, Liu FS (2011) Identification of two metallothionein genes and their roles in stress responses of Musca domestica toward hyperthermy and cadmium tolerance. Comp Biochem Physiol B: Biochem Mol Biol 160(2–3):81–88
Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M, Eckschlager T, Kizek R (2013) The role of metallothionein in oxidative stress. Int J Mol Sci 14(3):6044–6066
Andrews GK (2000) Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol 59(1):95–104
Lichtlen P, Schaffner W (2001) Putting its fingers on stressful situations: the heavy metal-regulatory transcription factor MTF-1. BioEssays 23(11):1010–1017
Paithankar JG, Saini S, Dwivedi S, Sharma A, Chowdhuri DK (2021) Heavy metal associated health hazards: an interplay of oxidative stress and signal transduction. Chemosphere 262:128350
Nair AR, DeGheselle O, Smeets K, Van Kerkhove E, Cuypers A (2013) Cadmium-induced pathologies: where is the oxidative balance lost (or not)? Int J Mol Sci 14(3):6116–6143
Corona M, Robinson GE (2006) Genes of the antioxidant system of the honey bee: annotation and phylogeny. Insect Mol Biol 15(5):687–701
Roh JY, Lee J, Choi J (2006) Assessment of stress-related gene expression in the heavy metal-exposed nematode Caenorhabditis elegans: a potential biomarker for metal-induced toxicity monitoring and environmental risk assessment. Environ Toxicol Chem Int J 25(11):2946–2956
Nair PMG, Park SY, Choi J (2011) Expression of catalase and glutathione S-transferase genes in Chironomus riparius on exposure to cadmium and nonylphenol. Comp Biochem Physiol C Toxicol Pharmacol 154(4):399–408
Park SY, Nair PMG, Choi J (2012) Characterization and expression of superoxide dismutase genes in Chironomus riparius (Diptera, Chironomidae) larvae as a potential biomarker of ecotoxicity. Comp Biochem Physiol C: Toxicol Pharmacol 156(3–4):187–194
Lee KW, Raisuddin S, Rhee JS, Hwang DS, Yu IT, Lee YM, Lee JS (2008) Expression of glutathione S-transferase (GST) genes in the marine copepod Tigriopus japonicus exposed to trace metals. Aquat Toxicol 89(3):158–166
Nair PMG, Choi J (2011) Identification, characterization and expression profiles of Chironomus riparius glutathione S-transferase (GST) genes in response to cadmium and silver nanoparticles exposure. Aquat Toxicol 101(3–4):550–560
Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC (2004) Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid Redox Signal 6(2):289–300
Xu ZB, Zou XP, Zhang N, Feng QL, Zheng SC (2015) Detoxification of insecticides, allechemicals and heavy metals by glutathione S-transferase SlGSTE1 in the gut of Spodoptera litura. Insect Sci 22(4):503–511
Honeybee Genome Sequencing Consortium (2006) Insights into social insects from the genome of the honeybee Apis mellifera. Nature 443(7114):931
Claudianos C, Ranson H, Johnson RM, Biswas S, Schuler MA, Berenbaum MR, Oakeshott JG (2006) A deficit of detoxification enzymes: pesticide sensitivity and environmental response in the honeybee. Insect Mol Biol 15(5):615–636
Fukuto TR (1990) Mechanism of action of organophosphorus and carbamate insecticides. Environ Health Perspect 87:245–254
Colovic MB, Krstic DZ, Lazarevic-Pasti TD, Bondzic AM, Vasic VM (2013) Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol 11(3):315–335
Lionetto MG, Caricato R, Calisi A, Giordano ME, Schettino T (2013) Acetylcholinesterase as a biomarker in environmental and occupational medicine: new insights and future perspectives. BioMed Res Int 2013
de Lima D, Roque GM, de Almeida EA (2013) In vitro and in vivo inhibition of acetylcholinesterase and carboxylesterase by metals in zebrafish (Danio rerio). Mar Environ Res 91:45–51
Carageorgiou H, Tzotzes V, Sideris A, Zarros A, Tsakiris S (2005) Cadmium effects on brain acetylcholinesterase activity and antioxidant status of adult rats: modulation by zinc, calcium and L-cysteine co-administration. Basic Clin Pharmacol Toxicol 97(5):320–324
Frasco MF, Fournier D, Carvalho F, Guilhermino L (2005) Do metals inhibit acetylcholinesterase (AChE)? Implementation of assay conditions for the use of AChE activity as a biomarker of metal toxicity. Biomarkers 10(5):360–375
Souid G, Souayed N, Yaktiti F, Maaroufi K (2013) Effect of acute cadmium exposure on metal accumulation and oxidative stress biomarkers of Sparus aurata. Ecotoxicol Environ Saf 89:1–7
Perić-Mataruga V, Petković B, Ilijin L, Mrdaković M, Čučaković SD, Todorović D, Vlahović M (2017) Cadmium and high temperature effects on brain and behaviour of Lymantria dispar L. caterpillars originating from polluted and less-polluted forests. Chemosphere 185:628–636
Liu Y, Chen Q, Li Y, Bi L, Jin L, Peng R (2022) Toxic effects of cadmium on fish. Toxics 10(10):622
Wang B, Du Y (2013) Cadmium and its neurotoxic effects. Oxidative Med Cell Longev 2013:898034
Andrade VM, Aschner M, Marreilha Dos Santos AP (2017) Neurotoxicity of metal mixtures. Neurotoxicity of metals 227–265
Collet C, Belzunces L (2007) Excitable properties of adult skeletal muscle fibres from the honeybee Apis mellifera. J Exp Biol 210(3):454–464
Quintavalle A (2013) Voltage-gated calcium channels in honey bees: physiological roles and potential targets for insecticides. BioSci Master Rev 2013:1–11
Rousset M, Collet C, Cens T, Bastin F, Raymond V, Massou I, Charnet P (2017) Honeybee locomotion is impaired by Am-CaV3 low voltage-activated Ca2+ channel antagonist. Sci Rep 7(1):1–10
Li Z, Qiu Y, Li J, Wan K, Nie H, Su S (2022) Chronic cadmium exposure induces impaired olfactory learning and altered brain gene expression in honey bees (Apis mellifera). Insects 13:988. https://doi.org/10.3390/insects13110988
Zarić NM, Ilijević K, Stanisavljević L, Gržetić I (2017) Use of honeybees (Apis mellifera L.) as bioindicators for assessment and source appointment of metal pollution. Environ Sci Pollut Res 24:25828–25838. https://doi.org/10.1007/s11356-017-0196-7
Funding
Open access funding provided by Università degli Studi di Sassari within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
S.K. and M.G.C. drafted and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Knoll, S., Cappai, M.G. Foraging Activity of Honey Bees (Apis mellifera L., 1758) and Exposure to Cadmium: a Review. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04118-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04118-3