Abstract
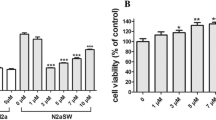
Selenium (Se) is an essential trace element for human health and plays an important role in the development and maintenance of central nervous system functions. Se deficiency has been associated with cognitive decline and increased oxidative stress. The increase in oxidative stress is one of the hypotheses for the emergence and worsening of neurodegenerative diseases, such as Alzheimer’s disease (AD). To investigate the neuroprotective effects of organic Se compounds in human neuroblastoma cells (SH-SY5Y) differentiated into cholinergic neurons-like. The SH-SY5Y cells were differentiated into cholinergic neuron-like with retinoic acid (RA) and brain-derived neurotrophic factor (BDNF). AD was mimicked exposing the cells to okadaic acid (OA) and beta-amyloid protein (Aβ). The neuroprotective effect of organic Se compounds, selenomethionine (SeMet) and Ebselen, was evaluated through cell viability tests, acetylcholinesterase and antioxidant enzyme activities, and detection of reactive oxygen species (ROS). None of the SeMet concentrations tested protected against the toxic effect of OA + Aβ. On the other hand, previous exposure to 0.1 and 1 µM Ebselen protected cells from the toxic effect of OA + Aβ. Cell differentiation induced by RA and BDNF exposure was effective, showing characteristics of neuronal cells, and pointing to a promising model of AD. Ebselen showed a protective effect, but more studies are needed to identify the mechanism of action.









Similar content being viewed by others
Data Availability
Enquiries about data availability should be directed to the authors.
References
Sharma K (2019) Cholinesterase inhibitors as Alzheimer’s therapeutics. Mol Med Rep 20:1479–1487. https://doi.org/10.3892/mmr.2019.10374
Oliveira CS, Piccoli BC, Nogara PA, Pereira ME, Carvalho KAT, Skalny AV, Tinkov AA, Aschner M, Rocha JBT (2021) Selenium neuroprotection in neurodegenerative disorders. In Handbook of Neurotoxicity, 2nd ed.; Kostrzewa RM, Ed.; Springer: Cham, Switzerland, 2021; Volume 1, p. 35. https://doi.org/10.1007/978-3-031-15080-7_238
Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J, van der Flier WM (2021) Alzheimer’s disease. Lancet 397:1577–1590. https://doi.org/10.1016/S0140-6736(20)32205-4
Fakhoury M (2018) Microglia and astrocytes in Alzheimer’s disease: implications for therapy. Curr Neuropharmacol 16:508–518. https://doi.org/10.2174/1570159X15666170720095240
Buchman AS, Bennett DA (2011) Loss of motor function in preclinical Alzheimer’s disease. Expert Rev Neurother 11:665–676. https://doi.org/10.1586/ern.11.57
Kueper JK, Lizotte DJ, Montero-Odasso M, Speechley M, Alzheimer’s Disease Neuroimaging Initiative (2020) Cognition and motor function: the gait and cognition pooled index. PLoS ONE 15(9):e0238690. https://doi.org/10.1371/journal.pone.0238690
Koenig AM, Arnold SE, Streim JE (2016) Agitation and irritability in Alzheimer’s Disease: evidenced-based treatments and the black-box warning. Curr Psychiatry Rep 18:3. https://doi.org/10.1007/s11920-015-0640-7
Dominiak A, Wilkaniec A, Wroczyński P, Adamczyk A (2016) Selenium in the therapy of neurological diseases. Where is it Going? Curr Neuropharmacol 14:282–299. https://doi.org/10.2174/1570159x14666151223100011
Pedrini S, Gupta VB, Hone E, Doecke J, O’Bryant S, James I, Bush AI, Rowe CC, Villemagne VL, Ames D, Masters CL, Martins RN, AIBL Research Group (2017) A blood-based biomarker panel indicates IL-10 and IL-12/23p40 are jointly associated as predictors of β-amyloid load in an AD cohort. Sci Rep 7:1–12. https://doi.org/10.1038/s41598-017-14020-9
Cardoso BR, Cominetti C, Cozzolino SM (2013) Importance and management of micronutrient deficiencies in patients with Alzheimer’s disease. Clin Interv Aging 8:531–542. https://doi.org/10.2147/CIA.S27983
Mehri A (2020) Trace elements in human nutrition (II) - an update. Int J Prev Med 11:2. https://doi.org/10.4103/ijpvm.IJPVM_48_19
Oliveira CS, Piccoli BC, Aschner M, Rocha JBT (2017) Chemical speciation of selenium and mercury as determinant of their neurotoxicity. In Neurotoxicity of Metals; Aschner M, Costa L, Eds.; Springer: Cham, Switzerland, Volume 18, pp. 53–83. https://doi.org/10.1007/978-3-319-60189-2_4
Rocha JBT, Piccoli BC, Oliveira CS (2017) Biological and chemical interest in selenium: a brief historical account. Arkivoc 457–491. https://doi.org/10.24820/ark.5550190.p009.784
Hassan W, Oliveira CS, Noreen H, Kamdem JP, Nogueira CW, Rocha JBT (2016) Organoselenium compounds as potential neuroprotective therapeutic agents. Curr Org Chem 20:218–231
Nogara PA, Oliveira CS, Rocha JBT (2020) Chemistry and pharmacology of synthetic organoselenium compounds. Organoselenium Chem., De Gruyter, Berlin, 305–346. https://doi.org/10.1515/9783110625110-008
Rocha JB, Oliveira CS, Nogara PA (2017) Toxicology and anticancer activity of synthetic organoselenium compounds. Organoselenium Compounds in Biology and Medicine. Synthesis, Biological and Therapeutic Treatments, pp 342–376
Cardoso BR, Bandeira VS, Jacob-Filho W, Cozzolino SMF (2014) Selenium status in elderly: relation to cognitive decline. J Trace Elem Med Biol 28:422–426. https://doi.org/10.1016/j.jtemb.2014.08.009
Cardoso BR, Apolinário D, Bandeira VS, Busse AL, Magaldi RM, Jacob-Filho W, Cozzolino SMF (2016) Effects of Brazil nut consumption on selenium status and cognitive performance in older adults with mild cognitive impairment: a randomized controlled pilot trial. Eur J Nutr 55:107–116. https://doi.org/10.1007/s00394-014-0829-2
Pereira ME, Souza JV, Galiciolli MEA, Sare F, Vieira GS, Kruk IL, Oliveira CS (2022) Effects of selenium supplementation in patients with mild cognitive impairment or Alzheimer’s Disease: a systematic review and meta-analysis. Nutrients 14:3205. https://doi.org/10.3390/nu14153205
Santi C, Scimmi C, Sancineto L (2021) Ebselen and Analogues: pharmacological properties and synthetic strategies for their preparation. Molecules 26:4230. https://doi.org/10.3390/molecules26144230
Nogara PA, Oliveira CS, Pereira ME, Bortoli M, Orian L, Aschner M, Rocha JBT (2022) Therapeutic applications of low-molecular-weight thiols and selenocompounds. In: Redox Chemistry and Biology of Thiols. Alvarez B, Comini MA, Salinas G, Trujillo M (Eds), Academic Press, Chap. 27, pp. 643–677. https://doi.org/10.1016/B978-0-323-90219-9.00005-4
Zhang ZH, Wu QY, Zheng R, Chen C, Chen Y, Liu Q, Hoffmann PR, Ni JZ, Song GL (2017) Selenomethionine mitigates cognitive decline by targeting both tau hyperphosphorylation and autophagic clearance in an Alzheimer’s disease mouse model. J Neurosci 37:2449–2462. https://doi.org/10.1523/JNEUROSCI.3229-16.2017
Martini F, Rosa SG, Klann IP, Fulco B, Carvalho FB, Rahmeier FL, Fernandes MC, Nogueira CW (2019) A multifunctional compound Ebselen reverses memory impairment, apoptosis and oxidative stress in a mouse model of sporadic Alzheimer’s disease. J Psychiatr Res 109:107–117. https://doi.org/10.1016/j.jpsychires.2018.11.021
Cogo SC, Nascimento TGFC, Pinhatti FAB, Junior NF, Rodrigues BS, Cavalli LR, Elifio-Esposito S (2020) An overview of neuroblastoma cell lineage phenotypes and in vitro models. Exp Biol Med 245:1637–1647. https://doi.org/10.1177/1535370220949237
Kovalevich J, Langford D (2013) Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol Biol 1078:9–21. https://doi.org/10.1007/978-1-62703-640-5_2
Medeiros LM, De Bastiani MA, Rico EP, Schonhofen P, Pfaffenseller B, Wollenhaupt-Aguiar B, Grun L, Barbé-Tuana F, Zimmer ER, Castro M, Parsons RB, Klamt F (2019) Cholinergic differentiation of human neuroblastoma SH-SY5Y cell line and its potential use as an in vitro model for Alzheimer’s Disease studies. Mol Neurobiol 56:7355–7367. https://doi.org/10.1007/s12035-019-1605-3
Lopes FM, Schröder R, da Frota ML Jr, Zanotto-Filho A, Müller CB, Pires AS, Meurer RT, Colpo GD, Gelain DP, Kapczinski F, Moreira JC, Fernandes M, Klamt F (2010) Comparison between proliferative and neuron-like SH-SY5Y cells as an in vitro model for Parkinson disease studies. Brain Res 1337:85–94. https://doi.org/10.1016/j.brainres.2010.03.102
Trzaska KA, Rameshwar P (2011) Dopaminergic neuronal differentiation protocol for human mesenchymal stem cells. Methods Mol Biol 698:295–303. https://doi.org/10.1007/978-1-60761-999-4_22
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Keen JH, Habig WH, Jakoby WB (1976) Mechanism for the several activities of the glutathione S-transferases. J Biol Chem 251:6183–6188
Gao R, Yuan Z, Zhao Z, Gao X (1998) Mechanism of pyrogallol autoxidation and determination of superoxide dismutase enzyme activity. Bioelect Bioenerg 45:41–45. https://doi.org/10.1016/S0302-4598(98)00072-5
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Tetz LM, Kamau PW, Cheng AA, Meeker JD, Loch-Caruso R (2013) Troubleshooting the dichlorofluorescein assay to avoid artifacts in measurement of toxicant-stimulated cellular production of reactive oxidant species. J Pharmacol Toxicol Methods 67:56–60. https://doi.org/10.1016/j.vascn.2013.01.195
Jämsä A, Hasslund K, Cowburn RF, Bäckström A, Vasänge M (2004) The retinoic acid and brain-derived neurotrophic factor differentiated SH-SY5Y cell line as a model for Alzheimer’s disease-like tau phosphorylation. Biochem Biophys Res Commun 319:993–1000. https://doi.org/10.1016/j.bbrc.2004.05.075
Shipley MM, Mangold CA, Szpara ML (2016) Differentiation of the SH-SY5Y human neuroblastoma cell line. J Vis Exp 108:53193. https://doi.org/10.3791/53193
Hromadkova L, Bezdekova D, Pala J, Schedin-Weiss S, Tjernberg LO, Hoschl C, Ovsepian SV (2020) Brain-derived neurotrophic factor (BDNF) promotes molecular polarization and differentiation of immature neuroblastoma cells into definitive neurons. Biochim Biophys Acta Mol Cell Res 1867:118737. https://doi.org/10.1016/j.bbamcr.2020.118737
Agholme L, Lindström T, Kågedal K, Marcusson J, Hallbeck M (2010) An in vitro model for neuroscience: differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J Alzheimer’s Dis 20:1069–1082. https://doi.org/10.3233/JAD-2010-091363
Duly A, Kao F, Teo WS, Kavallaris M (2022) βIII-tubulin gene regulation in health and disease. Front Cell Dev Biol 10:851542. https://doi.org/10.3389/fcell.2022.851542
Kamat PK, Nath C (2015) Okadaic acid: a tool to study regulatory mechanisms for neurodegeneration and regeneration in Alzheimer’s disease. Neural Regen Res 10:365–367. https://doi.org/10.4103/1673-5374.153679
Rostami J, Mothes T, Kolahdouzan M, Eriksson O, Moslem M, Bergström J, Ingelsson M, O’Callaghan P, Healy LM, Falk A, Erlandsson A (2021) Crosstalk between astrocytes and microglia results in increased degradation of α-synuclein and amyloid-β aggregates. J Neuroinflammation 18:124. https://doi.org/10.1186/s12974-021-02158-3
Kamat PK, Tota S, Rai S, Shukla R, Ali S, Najmi AK, Nath C (2012) Okadaic acid induced neurotoxicity leads to central cholinergic dysfunction in rats. Eur J Pharmacol 690:90–98. https://doi.org/10.1016/j.ejphar.2012.06.006
Kaushal A, Wani WY, Bal A, Gill KD, Kaur J (2019) Okadaic acid and hypoxia-induced dementia model of Alzheimer’s type in rats. Neurotox Res 35:621–634. https://doi.org/10.1007/s12640-019-0005-9
Zhang ZH, Wu QY, Zheng R, Chen C, Chen Y, Liu Q, Hoffmann PR, Ni JZ, Song GL (2017) Selenomethionine mitigates Cognitive decline by targeting both tau hyperphosphorylation and autophagic clearance in an Alzheimer’s Disease Mouse Model. J Neurosci 37(9):2449–2462. https://doi.org/10.1523/JNEUROSCI.3229-16.2017
Li X, Shi Q, Xu H, Xiong Y, Wang C, Le L, Lian J, Wu G, Peng F, Liu Q, Du X (2022) Ebselen Interferes with Alzheimer’s Disease by Regulating Mitochondrial Function. Antioxidants (Basel) 11(7):1350. Published 2022 Jul 11. https://doi.org/10.3390/antiox11071350
Nogara PA, Pereira ME, Orian L, Oliveira CS, Rocha JBT (2023) The Long Story of Ebselen: From About One Century of its Synthesis to Clinical Trials. In: Vito Lippolis; Claudio Santi; Eder J. Lenardão; Antonio L. Braga. (Org.). The Long Story of Ebselen: From About One Century of its Synthesis to Clinical Trials. 1ed. p. 567–591
Klann IP, Martini F, Rosa SG, Nogueira CW (2020) Ebselen reversed peripheral oxidative stress induced by a mouse model of sporadic Alzheimer’s disease. Mol Biol Rep 47(3):2205–2215. https://doi.org/10.1007/s11033-020-05326-5
Zhu W, Liu Y, Zhang W, Fan W, Wang S, Gu JH, Sun H, Liu F (2021) Selenomethionine protects hematopoietic stem/progenitor cells against cobalt nanoparticles by stimulating antioxidant actions and DNA repair functions. Aging 13:11705–11726. https://doi.org/10.18632/aging.202865
Reyes L, Bishop DP, Hawkins CL, Rayner BS (2019) Assessing the efficacy of Dietary Selenomethionine supplementation in the setting of Cardiac Ischemia/Reperfusion Injury. Antioxid (Basel Switzerland) 8:546. https://doi.org/10.3390/antiox8110546
Kajander EO, Harvima RJ, Kauppinen L, Akerman KK, Martikainen H, Pajula RL, Kärenlampi SO (1990) Effects of selenomethionine on cell growth and on S-adenosylmethionine metabolism in cultured malignant cells. Biochem J 267:767–774. https://doi.org/10.1042/bj2670767
Wambi CO, Sanzari JK, Sayers CM, Nuth M, Zhou Z, Davis J, Finnberg N, Lewis-Wambi JS, Ware JH, El-Deiry WS, Kennedy AR (2009) Protective effects of dietary antioxidants on proton total-body irradiation-mediated hematopoietic cell and animal survival. Radiat Res 172:175–186. https://doi.org/10.1667/RR1708.1
Nuth M, Kennedy AR (2013) Mitigating effects of L-selenomethionine on low-dose iron ion radiation-induced changes in gene expression associated with cellular stress. Oncol Lett 6:35–42. https://doi.org/10.3892/ol.2013.1362
Costa NS, Lima LS, Oliveira FAM, Galiciolli MEA, Manzano MI, Garlet QI, Irioda AC, Oliveira CS (2023) Antiproliferative Effect of Inorganic and Organic Selenium Compounds in breast cell lines. Biomedicines 11:1346. https://doi.org/10.3390/biomedicines11051346
Shi H, Liu S, Miyake M, Liu KJ (2006) Ebselen induced C6 glioma cell death in oxygen and glucose deprivation. Chem Res Toxicol 19:655–660. https://doi.org/10.1021/tx0502544
Song G, Zhang Z, Wen L, Chen C, Shi Q, Zhang Y, Ni J, Liu Q (2014) Selenomethionine ameliorates cognitive decline, reduces tau hyperphosphorylation, and reverses synaptic deficit in the triple transgenic mouse model of Alzheimer’s disease. J Alzheimer’s Dis 41:85–99. https://doi.org/10.3233/JAD-131805
Burk RF, Hill KE (2009) Selenoprotein P-expression, functions, and roles in mammals. Biochim Biophys Acta 1790:1441–1447. https://doi.org/10.1016/j.bbagen.2009.03.026
Xie Y, Tan Y, Zheng Y, Du X, Liu Q (2017) Ebselen ameliorates β-amyloid pathology, tau pathology, and cognitive impairment in triple-transgenic Alzheimer’s disease mice. J Biol Inorg Chem 22:851–865. https://doi.org/10.1007/s00775-017-1463-2
Li X, Shi Q, Xu H, Xiong Y, Wang C, Le L, Lian J, Wu G, Peng F, Liu Q, Du X (2022) Ebselen interferes with Alzheimer’s disease by regulating mitochondrial function. Antioxidants 11:1350. https://doi.org/10.3390/antiox11071350
Hampel H, Mesulam MM, Cuello AC, Farlow MR, Giacobini E, Grossberg GT, Khachaturian AS, Vergallo A, Cavedo E, Snyder PJ, Khachaturian ZS (2018) The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 141:1917–1933. https://doi.org/10.1093/brain/awy132
Farzi MA, Sadigh-Eteghad S, Ebrahimi K, Talebi M (2019) Exercise improves recognition memory and acetylcholinesterase activity in the beta amyloid-induced rat model of Alzheimer’s disease. Ann Neurosci 25:121–125. https://doi.org/10.1159/000488580
Campanari ML, García-Ayllón MS, Blazquez-Llorca L, Luk WK, Tsim K, Sáez-Valero J (2014) Acetylcholinesterase protein level is preserved in the Alzheimer’s brain. J Mol Neurosci 53:446–453. https://doi.org/10.1007/s12031-013-0183-5
García-Ayllón MS, Small DH, Avila J, Sáez-Valero J (2011) Revisiting the role of acetylcholinesterase in Alzheimer’s disease: cross-talk with P-tau and β-Amyloid. Front Mol Neurosci 4:22. https://doi.org/10.3389/fnmol.2011.00022
Melo JB, Agostinho P, Oliveira CR (2003) Involvement of oxidative stress in the enhancement of acetylcholinesterase activity induced by amyloid beta-peptide. Neurosci Res 45(1):117–127. https://doi.org/10.1016/s0168-0102(02)00201-8
Martini F, Bruning CA, Soares SM, Nogueira CW, Zeni G (2015) Inhibitory effect of ebselen on cerebral acetylcholinesterase activity in vitro: kinetics and reversibility of inhibition. Curr Pharm Des 21:920–924. https://doi.org/10.2174/1381612820666141014124319
Aras M, Altaş M, Meydan S, Nacar E, Karcıoğlu M, Ulutaş KT, Serarslan Y (2014) Effects of ebselen on ischemia/reperfusion injury in rat brain. Int J Neurosci 124(10):771–776. https://doi.org/10.3109/00207454.2013.879581
Marcus DL, Thomas C, Rodriguez C, Simberkoff K, Tsai JS, Strafaci JA, Freedman ML (1998) Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer’s disease. Exp Neurol 150:40–44. https://doi.org/10.1006/exnr.1997.6750
Angelucci F, Baiocco P, Brunori M, Gourlay L, Morea V, Bellelli A (2005) Insights into the catalytic mechanism of glutathione S-transferase: the lesson from Schistosoma haematobium. Structure 13:1241–1246. https://doi.org/10.1016/j.str.2005.06.007
Mazzetti AP, Fiorile MC, Primavera A, Lo Bello M (2015) Glutathione transferases and neurodegenerative diseases. Neurochem Int 82:10–18. https://doi.org/10.1016/j.neuint.2015.01.008
Ianiski FR, Alves CB, Souza AC, Pinton S, Roman SS, Rhoden CR, Alves MP, Luchese C (2012) Protective effect of meloxicam-loaded nanocapsules against amyloid-β peptide-induced damage in mice. Behav Brain Res 230(1):100–107. https://doi.org/10.1016/j.bbr.2012.01.055
Wilhelm EA, Bortolatto CF, Jesse CR, Luchese C (2014) Ebselen protects against behavioral and biochemical toxicities induced by 3-nitropropionic acid in rats: correlations between motor coordination, reactive species levels, and succinate dehydrogenase activity. Biol Trace Elem Res 162(1–3):200–210. https://doi.org/10.1007/s12011-014-0137-y
Lubos E, Loscalzo J, Handy DE (2011) Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 15:1957–1997. https://doi.org/10.1089/ars.2010.3586
Unsal C, Oran M, Albayrak Y, Aktas C, Erboga M, Topcu B, Uygur R, Tulubas F, Yanartas O, Ates O, Ozen OA (2016) Neuroprotective effect of ebselen against intracerebroventricular streptozotocin-induced neuronal apoptosis and oxidative stress in rats. Toxicol Ind Health 32(4):730–740. https://doi.org/10.1177/0748233713509429
Garlet QI, Haskel MVL, Pereira RP, da Silva WCFN, da Rocha JBT, Oliveira CS, Bonini JS (2019) Delta-aminolevulinate dehydratase and glutathione peroxidase activity in Alzheimer’s disease: a case-control study. EXCLI J 18:866–875. https://doi.org/10.17179/excli2019-1749
Martorell P, Bataller E, Llopis S, Gonzalez N, Alvarez B, Montón F, Ortiz P, Ramón D, Genovés S (2013) A cocoa peptide protects Caenorhabditis elegans from oxidative stress and β-amyloid peptide toxicity. PLoS ONE 8:e63283. https://doi.org/10.1371/journal.pone.0063283
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001 and the Instituto de Pesquisa Pelé Pequeno Príncipe (IPPPP).
Funding
Not applied.
Author information
Authors and Affiliations
Contributions
Study conception and design: MEP, ACI, and CSO; data collection: MEP and CSO; analysis and interpretation of results: MEP, LSL, NSC, JVS, JFS, ICG, and CSO; draft manuscript preparation: MEP, LSL, NSC, ACI, and CSO.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pereira, M.E., Lima, L.S., Souza, J.V. et al. Evaluation of the Neuroprotective Effect of Organic Selenium Compounds: An in Vitro Model of Alzheimer’s Disease. Biol Trace Elem Res 202, 2954–2965 (2024). https://doi.org/10.1007/s12011-023-03893-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03893-9