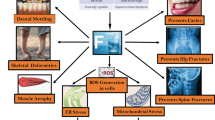
Abstract
Fluoride is present everywhere in nature. The primary way that individuals are exposed to fluoride is by drinking water. It’s interesting to note that while low fluoride levels are good for bone and tooth growth, prolonged fluoride exposure is bad for human health. Additionally, preclinical studies link oxidative stress, inflammation, and programmed cell death to fluoride toxicity. Moreover, mitochondria play a crucial role in the production of reactive oxygen species (ROS). On the other hand, little is known about fluoride’s impact on mitophagy, biogenesis, and mitochondrial dynamics. These actions control the growth, composition, and organisation of mitochondria, and the purification of mitochondrial DNA helps to inhibit the production of reactive oxygen species and the release of cytochrome c, which enables cells to survive the effects of fluoride poisoning. In this review, we discuss the different pathways involved in mitochondrial toxicity and dysfunction induced by fluoride. For therapeutic approaches, we discussed different phytochemical and pharmacological agents which reduce the toxicity of fluoride via maintained by imbalanced cellular processes, mitochondrial dynamics, and scavenging the ROS.






Similar content being viewed by others
Data Availability
We collected all the data from indexed journal and available online.
References
Stangvaltaite-Mouhat L et al (2021) Fluoride in the drinking water and dental caries experience by tooth surface susceptibility among adults. BMC Oral Health 21(1):234
Davoudi M et al (2021) Relationship of fluoride in drinking water with blood pressure and essential hypertension prevalence: a systematic review and meta-analysis. Int Arch Occup Environ Health 94(6):1137–1146
Srivastava S, Flora SJS (2020) Fluoride in drinking water and skeletal fluorosis: a review of the global impact. Curr Environ Health Rep 7(2):140–146
Liang C et al (2020) Fluoride induced mitochondrial impairment and PINK1-mediated mitophagy in Leydig cells of mice: In vivo and in vitro studies. Environ Pollut 256:113438
Ge QD et al (2019) Differential expression of miRNAs in the hippocampi of offspring rats exposed to fluorine combined with aluminum during the embryonic stage and into adulthood. Biol Trace Elem Res 189(2):463–477
Tang Z et al (2021) Mangiferin prevents the impairment of mitochondrial dynamics and an increase in oxidative stress caused by excessive fluoride in SH-SY5Y cells. J Biochem Mol Toxicol 35(4):e22705
Herath H, Kawakami T, Tafu M (2018) Repeated heat regeneration of bone char for sustainable use in fluoride removal from drinking water. Healthcare (Basel) 6(4)
Urbansky ET (2002) Fate of fluorosilicate drinking water additives. Chem Rev 102(8):2837–2854
Khichar M, Kumbhat S (2015) Defluoridation-a review of water from aluminium and alumina based compound. International Journal of chemical studies 2:04–11
Dhar V, Bhatnagar M (2009) Physiology and toxicity of fluoride. Indian J Dent Res 20(3):350–355
O'Mullane DM et al (2016) Fluoride and oral health. Community Dent Health 33(2):69–99
Kanduti D, Sterbenk P, Artnik B (2016) Fluoride: a review of use and effects on health. Mater Soc 28(2):133–137
Ameeramja J, Perumal E (2018) Possible modulatory effect of tamarind seed coat extract on fluoride-induced pulmonary inflammation and fibrosis in rats. Inflammation 41(3):886–895
Ameeramja J, Perumal E (2017) Pulmonary fluorosis: a review. Environ Sci Pollut Res Int 24(28):22119–22132
Kanagaraj VV et al (2015) Caffeic acid, a phyto polyphenol mitigates fluoride induced hepatotoxicity in rats: a possible mechanism. Biofactors 41(2):90–100
Ekambaram P et al (2010) Therapeutic efficacy of Tamarindus indica (L) to protect against fluoride-induced oxidative stress in the liver of female rats. Fluoride 43:134–140
Panneerselvam L, Raghunath A, Perumal E (2017) Acute fluoride poisoning alters myocardial cytoskeletal and AMPK signaling proteins in rats. Int J Cardiol 229:96–101
Panneerselvam L et al (2019) Acute fluoride exposure alters myocardial redox and inflammatory markers in rats. Mol Biol Rep 46(6):6155–6164
Dharmaratne RW (2019) Exploring the role of excess fluoride in chronic kidney disease: a review. Hum Exp Toxicol 38(3):269–279
Podder S et al (2010) Histopathology and cell cycle alteration in the spleen of mice from low and high doses of sodium fluoride. Fluoride 43:237–245
Paul V, Ekambaram P, Jayakumar AR (1998) Effects of sodium fluoride on locomotor behavior and a few biochemical parameters in rats. Environ Toxicol Pharmacol 6(3):187–191
Ekambaram P, Paul V (2002) Modulation of fluoride toxicity in rats by calcium carbonate and by withdrawal of fluoride exposure. Pharmacol Toxicol 90(2):53–58
Bhatnagar M et al (2011) Effects of fluoride in drinking water on NADPH-diaphorase neurons in the forebrain of mice: a possible mechanism of fluoride neurotoxicity. Fluoride 44:195–209
Niu R et al (2015) Effects of fluoride on microtubule ultrastructure and expression of Tubα1a and Tubβ2a in mouse hippocampus. Chemosphere 139:422–427
Shalini B, Sharma JD (2015) Beneficial effects of Emblica officinalis on fluoride-induced toxicity on brain biochemical indexes and learning-memory in rats. Toxicol Int 22(1):35–39
Madhusudhan N et al (2010) Effect of maternal fluoride exposure on developing CNS of rats: protective role of Aloe vera, Curcuma longa and Ocimum sanctum. Indian J Exp Biol 48(8):830–836
Needham LL et al (2011) Partition of environmental chemicals between maternal and fetal blood and tissues. Environ Sci Technol 45(3):1121–1126
Vani M, Reddy K (2000) Effects of fluoride accumulation on some enzymes of brain and gastrocnemius muscle of mice. Fluoride 33
Shivarajashankara YM et al (2002) Histological changes in the brain of young fluoride-intoxicated rats. Fluoride 35:12–21
Whitford GM (1994) Intake and metabolism of fluoride. Adv Dent Res 8(1):5–14
Barbier O, Arreola-Mendoza L, Del Razo LM (2010) Molecular mechanisms of fluoride toxicity. Chem Biol Interact 188(2):319–333
Kubota K et al (2005) Fluoride induces endoplasmic reticulum stress in ameloblasts responsible for dental enamel formation. J Biol Chem 280(24):23194–23202
Yan Q et al (2007) Micromolar fluoride alters ameloblast lineage cells in vitro. J Dent Res 86(4):336–340
Jacinto-Alemán LF et al (2010) In vitro effect of sodium fluoride on antioxidative enzymes and apoptosis during murine odontogenesis. J Oral Pathol Med 39(9):709–714
Karube H et al (2009) NaF activates MAPKs and induces apoptosis in odontoblast-like cells. J Dent Res 88(5):461–465
Qu WJ et al (2008) Sodium fluoride modulates caprine osteoblast proliferation and differentiation. J Bone Miner Metab 26(4):328–334
Yan X et al (2009) Effects of sodium fluoride treatment in vitro on cell proliferation, apoptosis and caspase-3 and caspase-9 mRNA expression by neonatal rat osteoblasts. Arch Toxicol 83(5):451–458
Yang S et al (2011) Sodium fluoride induces apoptosis and alters bcl-2 family protein expression in MC3T3-E1 osteoblastic cells. Biochem Biophys Res Commun 410(4):910–915
Wyllie AH (2010) “Where, O death, is thy sting?” A brief review of apoptosis biology. Mol Neurobiol 42(1):4–9
Ola MS, Nawaz M, Ahsan H (2011) Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem 351(1-2):41–58
Mason EF, Rathmell JC (2011) Cell metabolism: an essential link between cell growth and apoptosis. Biochim Biophys Acta 1813(4):645–654
Ganeshan K, Chawla A (2014) Metabolic regulation of immune responses. Annu Rev Immunol 32:609–634
O'Neill LA, Kishton RJ, Rathmell J (2016) A guide to immunometabolism for immunologists. Nat Rev Immunol 16(9):553–565
Fina BL et al (2014) Fluoride increases superoxide production and impairs the respiratory chain in ROS 17/2.8 osteoblastic cells. PLoS One 9(6):e100768
Li QS et al (2017) Effect of fluoride treatment on gene expression in tea plant (Camellia sinensis). Sci Rep 7(1):9847
Kim JW et al (2021) Blood hemoglobin, in-vivo Alzheimer pathologies, and cognitive impairment: a cross-sectional study. Front Aging Neurosci 13:625511
Gassowska M et al (2013) Effect of fluoride on sodium-proton exchanger activity, intracellular pH and calcium concentration in human non-stimulated platelets. Ann Acad Med Stetin 59(2):54–61
Cai JN et al (2018) Sucrose challenges to Streptococcus mutans biofilms and the curve fitting for the biofilm changes. FEMS Microbiol Ecol 94(7)
Han Y (2021) Effects of brief sodium fluoride treatments on the growth of early and mature cariogenic biofilms. Sci Rep 11(1):18290
Wei M et al (2022) Effect of fluoride on cytotoxicity involved in mitochondrial dysfunction: a review of mechanism. Front Vet Sci 9:850771
Landry J et al (2000) The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci U S A 97(11):5807–5811
Imai S et al (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403(6771):795–800
Smith JS et al (2000) A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc Natl Acad Sci U S A 97(12):6658–6663
Blander G, Guarente L (2004) The Sir2 family of protein deacetylases. Annu Rev Biochem 73:417–435
Morris BJ (2013) Seven sirtuins for seven deadly diseases of aging. Free Radic Biol Med 56:133–171
Frye RA (2000) Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun 273(2):793–798
Beausoleil SA et al (2004) Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc Natl Acad Sci U S A 101(33):12130–12135
Sasaki T et al (2008) Phosphorylation regulates SIRT1 function. PLoS One 3(12):e4020
Nasrin N et al (2009) JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS One 4(12):e8414
Kume S et al (2010) Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest 120(4):1043–1055
Hasegawa K et al (2008) Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem Biophys Res Commun 372(1):51–56
Ghosh HS, Reizis B, Robbins PD (2011) SIRT1 associates with eIF2-alpha and regulates the cellular stress response. Sci Rep 1:150
Han MK et al (2008) SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell 2(3):241–251
Hariharan N et al (2010) Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ Res 107(12):1470–1482
Lee IH et al (2008) A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A 105(9):3374–3379
Ohsumi Y (2001) Molecular dissection of autophagy: two ubiquitin-like systems. Nat Rev Mol Cell Biol 2(3):211–216
Shintani T, Klionsky DJ (2004) Autophagy in health and disease: a double-edged sword. Science 306(5698):990–995
Lum JJ, DeBerardinis RJ, Thompson CB (2005) Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol 6(6):439–448
Wei G et al (2021) Sirtuin 1 alleviates neuroinflammation-induced apoptosis after traumatic brain injury. J Cell Mol Med 25(9):4478–4486
Xu D et al (2019) SIRT2 functions in aging, autophagy, and apoptosis in post-maturation bovine oocytes. Life Sci 232:116639
Tu LF et al (2019) Sirt3-dependent deacetylation of COX-1 counteracts oxidative stress-induced cell apoptosis. FASEB J 33(12):14118–14128
Wang Y et al (2018) Sirtuin 5 overexpression attenuates glucolipotoxicity-induced pancreatic β cells apoptosis and dysfunction. Exp Cell Res 371(1):205–213
Liu M et al (2017) Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat Commun 8(1):413
Vakhrusheva O et al (2008) Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res 102(6):703–710
Li F et al (2015) NADP(+)-IDH mutations promote hypersuccinylation that impairs mitochondria respiration and induces apoptosis resistance. Mol Cell 60(4):661–675
Suzuki M, Bartlett JD (2014) Sirtuin1 and autophagy protect cells from fluoride-induced cell stress. Biochim Biophys Acta 1842(2):245–255
Nogueiras R et al (2012) Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev 92(3):1479–1514
Hayashida S et al (2010) Fasting promotes the expression of SIRT1, an NAD+ -dependent protein deacetylase, via activation of PPARalpha in mice. Mol Cell Biochem 339(1-2):285–292
Han L et al (2010) SIRT1 is regulated by a PPAR {γ}-SIRT1 negative feedback loop associated with senescence. Nucleic Acids Res 38(21):7458–7471
Noriega LG et al (2011) CREB and ChREBP oppositely regulate SIRT1 expression in response to energy availability. EMBO Rep 12(10):1069–1076
McCubrey JA et al (2017) Effects of resveratrol, curcumin, berberine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs. Aging (Albany NY) 9(6):1477–1536
Halliwell B, Cross CE (1994) Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect 102(Suppl 10):5–12
Ames BN, Shigenaga MK, Hagen TM (1993) Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A 90(17):7915–7922
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552(Pt 2):335–344
Andreyev AY, Kushnareva YE, Starkov AA (2005) Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 70(2):200–214
Starkov AA et al (2004) Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci 24(36):7779–7788
Tretter L, Adam-Vizi V (2004) Generation of reactive oxygen species in the reaction catalyzed by alpha-ketoglutarate dehydrogenase. J Neurosci 24(36):7771–7778
Migliaccio E, Giorgio M, Pelicci PG (2006) Apoptosis and aging: role of p66Shc redox protein. Antioxid Redox Signal 8(3-4):600–608
Korshunov SS, Skulachev VP, Starkov AA (1997) High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416(1):15–18
Lambert AJ, Brand MD (2004) Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem J 382(Pt 2):511–517
Circu ML et al (2009) Contribution of glutathione status to oxidant-induced mitochondrial DNA damage in colonic epithelial cells. Free Radic Biol Med 47(8):1190–1198
Rachek LI et al (2009) Troglitazone, but not rosiglitazone, damages mitochondrial DNA and induces mitochondrial dysfunction and cell death in human hepatocytes. Toxicol Appl Pharmacol 240(3):348–354
Ricci C et al (2008) Mitochondrial DNA damage triggers mitochondrial-superoxide generation and apoptosis. Am J Phys Cell Phys 294(2):C413–C422
Fritz R et al (2007) Compartment-dependent management of H (2)O(2) by peroxisomes. Free Radic Biol Med 42(7):1119–1129
Zangar RC, Davydov DR, Verma S (2004) Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol Appl Pharmacol 199(3):316–331
Caro AA, Cederbaum AI (2006) Role of cytochrome P450 in phospholipase A2- and arachidonic acid-mediated cytotoxicity. Free Radic Biol Med 40(3):364–375
Dumitru CA et al (2007) Ceramide: a novel player in reactive oxygen species-induced signaling? Antioxid Redox Signal 9(9):1535–1540
Woo CC et al (2013) Thymoquinone inhibits tumor growth and induces apoptosis in a breast cancer xenograft mouse model: the role of p38 MAPK and ROS. PLoS One 8(10):e75356
Dai X et al (2017) A novel benzimidazole derivative, MBIC inhibits tumor growth and promotes apoptosis via activation of ROS-dependent JNK signaling pathway in hepatocellular carcinoma. Oncotarget 8(8):12831–12842
Kim C et al (2018) Formononetin-induced oxidative stress abrogates the activation of STAT3/5 signaling axis and suppresses the tumor growth in multiple myeloma preclinical model. Cancer Lett 431:123–141
Giorgio M et al (2005) Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122(2):221–233
Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116(2):205–219
Simon HU, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis : an international journal on programmed cell death 5:415–418
Redza-Dutordoir M, Averill-Bates DA (2016) Activation of apoptosis signalling pathways by reactive oxygen species. Biochimica et Biophysica Acta (BBA) - Molecular. Cell Res 1863(12):2977–2992
Giorgio M et al (2007) Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol 8(9):722–728
Salar RK, Seasotiya L (2011) Free radical scavenging activity, phenolic contents and phytochemical evaluation of different extracts of stem bark of Butea monosperma (Lam.) Kuntze. Frontiers in Life Science 5(3-4):107–116
Benmehdi H et al (2017) Free radical scavenging activity, kinetic behaviour and phytochemical constituents of Aristolochia clematitis L. roots. Arab J Chem 10:S1402–S1408
Thatoi HN, Patra JK, Das SK (2014) Free radical scavenging and antioxidant potential of mangrove plants: a review. Acta Physiol Plant 36(3):561–579
Gad FA, Farouk SM, Emam MA (2021) Antiapoptotic and antioxidant capacity of phytochemicals from Roselle (Hibiscus sabdariffa) and their potential effects on monosodium glutamate-induced testicular damage in rat. Environ Sci Pollut Res Int 28(2):2379–2390
Dutta K, Ghosh D, Basu A (2009) Curcumin protects neuronal cells from Japanese encephalitis virus-mediated cell death and also inhibits infective viral particle formation by dysregulation of ubiquitin-proteasome system. J NeuroImmune Pharmacol 4(3):328–337
Sobeh M et al (2017) Senna singueana: antioxidant, hepatoprotective, antiapoptotic properties and phytochemical profiling of a methanol bark extract. Molecules 22(9)
Choi YJ et al (2003) Polyphenolic flavonoids differ in their antiapoptotic efficacy in hydrogen peroxide-treated human vascular endothelial cells. J Nutr 133(4):985–991
Inkielewicz-Stepniak I, Radomski MW, Wozniak M (2012) Fisetin prevents fluoride- and dexamethasone-induced oxidative damage in osteoblast and hippocampal cells. Food Chem Toxicol 50(3-4):583–589
Sujatha K (2016) Hematobiochemical and antioxidant evaluation of Aloe vera whole leaf extract on fluoride induced toxicity in Wistar albino rats. SOJ Veterinary Sciences 2(1):1–5
Bouasla A et al (2016) Prophylactic effects of pomegranate (Punica granatum) juice on sodium fluoride induced oxidative damage in liver and erythrocytes of rats. Can J Physiol Pharmacol 94(7):709–718
Scherz-Shouval R, Shvets E, Elazar Z (2007) Oxidation as a post-translational modification that regulates autophagy. Autophagy 3(4):371–373
Scherz-Shouval R, Elazar Z (2011) Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci 36:30–38
Ferro F et al (2020) Autophagy and mitophagy in cancer metabolic remodelling. Semin Cell Dev Biol 98:129–138
Scherz-Shouval R, Elazar Z (2007) ROS, mitochondria and the regulation of autophagy. Trends Cell Biol 17(9):422–427
Ciccarone F, Castelli S, Ciriolo MR (2019) Oxidative stress-driven autophagy across onset and therapeutic outcome in hepatocellular carcinoma. Oxidative Med Cell Longev 2019:6050123
Azad MB, Chen Y, Gibson SB (2009) Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal 11(4):777–790
Lim SD et al (2005) Increased Nox1 and hydrogen peroxide in prostate cancer. Prostate 62(2):200–207
Ghavami S et al (2008) Brevinin-2R(1) semi-selectively kills cancer cells by a distinct mechanism, which involves the lysosomal-mitochondrial death pathway. J Cell Mol Med 12(3):1005–1022
Cai J et al (2008) Emodin-induced generation of reactive oxygen species inhibits RhoA activation to sensitize gastric carcinoma cells to anoikis. Neoplasia 10(1):41–51
Poillet-Perez L et al (2015) Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol 4:184–192
Rodríguez-Vargas JM, Oliver-Pozo FJ, Dantzer F (2019) PARP1 and poly (ADP-ribosyl) ation signaling during autophagy in response to nutrient deprivation. Oxidative Med Cell Longev 2019:2641712
Boyer-Guittaut M et al (2014) The role of GABARAPL1/GEC1 in autophagic flux and mitochondrial quality control in MDA-MB-436 breast cancer cells. Autophagy 10(6):986–1003
Lin Y et al (2019) Cancer and ER stress: Mutual crosstalk between autophagy, oxidative stress and inflammatory response. Biomed Pharmacother 118:109249
Kim YS et al (2007) TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell 26(5):675–687
Li L et al (2015) ROS and autophagy: interactions and molecular regulatory mechanisms. Cell Mol Neurobiol 35(5):615–621
Adebayo M et al (2021) Mitochondrial fusion and fission: the fine-tune balance for cellular homeostasis. FASEB J 35(6):e21620
Puty B et al (2021) Human cultured IMR-32 neuronal-like and U87 glial-like cells have different patterns of toxicity under fluoride exposure. PLoS One 16(6):e0251200
Di Pietro V et al (2017) Fusion or fission: the destiny of mitochondria in traumatic brain injury of different severities. Sci Rep 7(1):9189
Oshima Y et al (2021) Parkin-independent mitophagy via Drp1-mediated outer membrane severing and inner membrane ubiquitination. J Cell Biol 220(6)
Lee JE et al (2016) Multiple dynamin family members collaborate to drive mitochondrial division. Nature 540(7631):139–143
Valera-Alberni M et al (2021) Crosstalk between Drp1 phosphorylation sites during mitochondrial remodeling and their impact on metabolic adaptation. Cell Rep 36(8):109565
Feng Z et al (2019) Effects of Fluoride on Autophagy in Mouse Sertoli Cells. Biol Trace Elem Res 187(2):499–505
Ommati MM et al (2018) Is immunosuppression, induced by neonatal thymectomy, compatible with poor reproductive performance in adult male rats? Andrology 6(1):199–213
Chen Y et al (2020) Mitochondrial fusion and fission in neuronal death induced by cerebral ischemia-reperfusion and its clinical application: a mini-review. Med Sci Monit 26:e928651
Zhou BH et al (2020) Drp1/Mff signaling pathway is involved in fluoride-induced abnormal fission of hepatocyte mitochondria in mice. Sci Total Environ 725:138192
Song B et al (2021) Methionine deficiency affects liver and kidney health, oxidative stress, and ileum mucosal immunity in broilers. Front Vet Sci 8:722567
AlRefeai MH et al (2021) Assessment of bond integrity, durability, and degree of conversion of a calcium fluoride reinforced dentin adhesive. Polymers (Basel) 13(15)
Aulestia FJ et al (2020) Fluoride exposure alters Ca(2+) signaling and mitochondrial function in enamel cells. Sci Signal 13(619)
Mohamed NE (2016) The role of calcium in ameliorating the oxidative stress of fluoride in rats. Biol Trace Elem Res 170(1):128–144
Lezmy J et al (2021) Astrocyte Ca(2+)-evoked ATP release regulates myelinated axon excitability and conduction speed. Science 374(6565):eabh2858
Data availability
All the data used from publicly available search engines namely PubMed, Scopus, Web of Science. These articles are available online.
Funding
We thank Indian Council of Medical Research for providing Extra mural research funding including fellowship support to Mr. Sachindra Kumar (Grant Number: - 5/8-4/6/Env/2020-NCD-II)
Author information
Authors and Affiliations
Contributions
Conceptualisation: Sachindra Kumar, Smita Shenoy, Ravindra Shantakumar Swamy, V. Ravichandiran, Nitesh Kumar. Manuscript writing: Sachindra Kumar, Nitesh Kumar. Figures: Sachindra Kumar, Nitesh Kumar. Review: Sachindra Kumar, Smita Shenoy, Ravindra Shantakumar Swamy, V. Ravichandiran, Nitesh Kumar
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, S., Shenoy, S., Swamy, R.S. et al. Fluoride-Induced Mitochondrial Dysfunction and Approaches for Its Intervention. Biol Trace Elem Res 202, 835–849 (2024). https://doi.org/10.1007/s12011-023-03720-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03720-1