Abstract
Muskox (Ovibos moschatus) and caribou (Rangifer tarandus groenlandicus) are wild ruminants that inhabit the Greenland tundra. They are part of the diet of many Greenlanders, being important sources of protein and micronutrients such as iron. The objective of this study is to analyse the element profiles of three tissues from these species: skeletal muscle, liver and adipose tissue, and to determine if they are affected by species and sex (male vs. female). Samples were obtained from annual hunts in two different regions of West Greenland. Element profiles were analysed using inductively-coupled plasma–optical emission spectrometry. The interaction between species and sex was only detected in Na (sodium) in the muscle and adipose tissue, where male and female caribou had the highest concentrations, respectively. The effect of sex was not statistically significant in the liver samples and only occasionally in the other tissues. Species was the most relevant factor in element profiles found in this study. Caribou had higher concentrations of K (potassium) and S (sulphur) in the muscle and liver. Fewer differences were detected between species in the adipose tissue, compared to the other tissues. These differences may reflect the feeding behaviour and the geographical location of both species. This study contributes to evaluate the element composition of the edible tissue of these wild ungulate species, as well as evaluating the factors of sex and species that could differentiate their composition.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
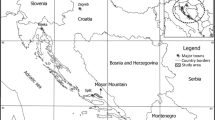
The Arctic has long been the habitat of two wild ungulate species: the muskox (Ovibos moschatus) and the caribou (Rangifer tarandus groenlandicus). These species co-exist in West Greenland in the Kangerlussuaq-Sisimiut (KS) region, while only caribou are found in the Akia-Maniitsoq (AM) region (Fig. 1). During the latest survey in 2018, the muskox population in the KS region was estimated at ca. 20.300 animals [1]. while caribou numbers in the KS and AM regions were estimated at ca. 98.300 animals and 24.000, respectively [2]. Both species need to accumulate nutrients during the spring and summer, when forage quality and quantity are the highest. The muskox is predominantly a grazer, whereas the caribou is an intermediate feeder [3]. In addition, the caribou feeds on lichens during the winter if they are available [3]. The muskox has developed a slower digestive process, maximizing feed intake, enabled by a large rumen compared to that of the caribou [4]. The element status of wild animals is influenced by forage availability and their feeding behaviour, ultimately influencing their health and meat quality [5].
Elements are of paramount importance for the maintenance of physiological functions. Herein, these will be divided in three classes: macroelements (e.g. sodium (Na), potassium (K), sulphur (S)), microelements (e.g. iron (Fe), zinc (Zn), copper (Cu)) and other non-essential elements (e.g. cadmium (Cd), arsenic (As) and lead (Pb)). Their functions range from maintaining acid–base balance to acting as key structural components of molecules and organisms [5]. Several factors influence element concentration in tissues, including nutrition, geographic location and sex. Feed restriction has been reported to increase element concentrations in the muscle, liver and adipose tissue of ram lambs (Ovis aries) [6, 7]. Sex influences the element concentration of tissues from large ruminant species, such as the yak (Bos grunniens) or water buffalo (Bubalus bubalis) [5]. The influence of nutrition and geographic location has been described in the liver and kidney tissues of Svalbard reindeer (Rangifer tarandus platyrhynchus), reflecting the impact of different dietary compositions in distinct populations [8]. In Canada, the concentrations of selenium (Se) in the liver and serum of muskox were found to be low, which was attributed to the geographic location and soil properties [9]. The objective of this study is to analyse the effect of sex and species on the element content of muscle, liver and fat of caribou and muskox from West Greenland. This will provide complementary information to existing literature on the effect of sex as well as information on elements (including contaminants) that may be present in such edible tissues, focusing on the two specific regions of Greenland.
Materials and Methods
-
a. Sample Collection
Procedures for sample harvesting have been described for the muskox samples [10, 11]. Briefly, muskoxen were hunted in the Kangerlussuaq-Sisimiut region, West Greenland during winter. Animals were eviscerated and bled on kill site and brought to the Kangerlussuaq butchery where samples were taken from the longissimus dorsi muscle, liver and subcutaneous adipose tissue of female (n = 12) and male (n = 8) muskoxen. Average carcass weight of the sampled animals was 76.0 ± 9.51 and 89.5 ± 19.87 kg for females and males, respectively. The caribou samples were harvested in a similar manner, during an annual summer hunt in the Akia-Maniitsoq region. Samples were taken from ten males and eight females on site. All samples were frozen, transported to the lab and freeze-dried for 72 h until constant weight using a Christ Alpha 1-2 LDplus freeze drier (Christ Alpha, Osterode am Harz, Germany). They were then shipped to Portugal for further analysis.
Samples from both species were obtained during the annual commercial hunting season following Greenland legislation. Animals were thus hunted for consumption and not for research purposes. As such, no ethical approval is necessary.
-
b. Element Analysis
The protocol for element analysis was previously described in Ribeiro et al. [6]. Briefly, approximately 0.3 g of dried sample was weighed into a digestion tube. For sample dissolution, hydrogen peroxide, nitric acid and hydrochloric acid — 1:3:10 (v/v/v) — were added. Each digestion set had a blank tube, without sample, to perform blank correction. The tubes were randomly distributed in a digestion plate (DigiPREP MS, SCP Science, Quebec, Canada) in which they were heated gradually for 1 h until reaching 95 °C and then at 95 °C constantly for another hour. Samples were then diluted to a total volume of 25 mL using distilled water and filtered using 90-mm filter paper (Filter-Lab ref. 1242, Filtros Anoia S.A., Barcelona, Spain).
Inductively coupled plasma–optical emission spectrometry (ICP-OES) readings were performed in a Thermo Scientific iCAP 7200 Duo spectrometer (Thermo Scientific, Waltham, MA, USA). Multi-element PlasmaQual S22 standards (SCP Science, Baie D’Urfé, QC, Canada) were used to create the calibration curves necessary to quantify the different elements (between 5 and 300 mg/L for Na, K, Ca, Mg, P and S and between 0.05 and 20 mg/L for the other elements). Multi-element detection and quantification took place overnight to detect the following 21 elements (limits of quantification, in mg/kg, are indicated after the name of the element): Sn (tin, 2.5), V (vanadium, 0.5), Li (lithium, 1.0), Ba (barium, 2.0), Se (selenium, 1.0), As (arsenic, 2.0), Co (cobalt, 1.0), Zn (zinc, 1.0), Fe (iron, 1.0), Mn (manganese, 1.0), Cu (copper, 1.0), Pb (lead, 0.5), Cd (cadmium, 0.05), Ni (nickel, 2.0), Cr (chromium, 2.0), S (sulphur, 50), P (phosphorous, 50), Mg (magnesium, 20), Ca (calcium, 20), K (potassium, 150) and Na (sodium, 150). Recovery values from spiked samples were always within a 10% range. Results were confirmed by quantifying the elements in certified reference materials (Wepal IPE 776) that underwent the same experimental procedure as the samples. Recovery values of the standards are reported in supplementary material 1. No further dilution was needed for any element before analysis. Results were calculated on a dry matter (DM) basis without correcting for lipid content.
-
c. Statistical Analysis
In total, 18 elements were identified in all tissues: Na, K, Ca, Mg, P, S, Cr, Cd, Pb, Cu, Mn, Fe, Zn, Co, As, Ba, Li and Sn. Three elements were identified in concentrations below the lower limit of quantification and were not considered for subsequent statistical analysis: Ni (< 2.0 mg/kg DM), Se (< 1.0 mg/kg DM) and V (< 0.5 mg/kg DM). Whenever a reading was found to be below the limit of quantification, it was considered as not acquired and was not used to calculate means. Analysis of variance was carried out using the GLM procedure of SAS system, 3rd edition (SAS Institute Inc.): species, sex and the interaction between the two factors were fitted. When significant p values (p < 0.05) of the interaction were obtained, least square means were compared using the Tukey test. The univariate procedure was used to obtain standard error of the means (SEM). A principal component analysis (PCA) was carried out using the prcomp function and the factoextra package in R Studio (version 3.6.2, The R Foundation for Statistical Computing) to visualize the first two components.
Results and Discussion
In this study, like others where samples are collected from field conditions, differences found with statistical significance may have a multitude of causes. There are differences inherent to each species: the caribou has more selective feeding habits, while the muskox feeds in bulk and has a larger rumen, comparatively increasing digestive efficiency of poor-quality forage [4].
Element concentrations of the muscle tissue are presented in Table 1, and the corresponding p-values for their effects are depicted in Table 2. At the species level, caribou had higher concentrations of K, Mg, P and S than muskox, reaching a 1.95-fold difference in S (p < 0.05). The contrary was observed for Ca, where muskox had higher concentrations. In the liver (Tables 3 and 4), all macroelements had statistically significant differences between species (p < 0.05). Muskox had higher concentrations of Na, Ca and Mg, whereas caribou had higher concentrations of K, P and S. Caribou had higher concentration of Mn compared to muskox, whereas the concentration of Cu had the inverse relationship. Element concentrations in the adipose tissue were generally lower and there were fewer significant effects, compared to the other two tissues (Tables 5 and 6). Caribou had higher concentrations of elements than muskox with significant differences, including Na, S, Mg, Zn, As and Cu (p < 0.05).
The differences mentioned above are corroborated by the PCA analysis performed (Fig. 2). Indeed, the effect of species is most pronounced in both the muscle and liver, with the adipose tissue having less discrimination between caribou and muskox. Distinction based on sex is not clearly recognizable.
The lower number of differences found in adipose tissue could point towards influence of muscle and hepatic lipid contents on those tissues’ profiles, given that fat has a diluting effect on tissue element profiles [7]. Excluding lipid content, the differences mentioned above could derive from multiple factors. Gamberg et al. [12] have analysed contaminants in caribou tissues from the KS and AM regions. According to these authors, in those regions, available forage is composed by monocots (KS) and lichens during winter (AM). Thus, in the KS area, muskoxen graze mostly on the available grasses, shrubs and other graminoids [13, 14]. In turn, the caribou of the present study can feed on lichens, which have been described to have a Ca concentration more than double the amount found in other feed sources such as Salix sp., upon which the muskox also rely [13, 15]. Lichen availability has been reported as being a major contributing factor for the element status of muscle and liver of the caribou [16]. It is expected that the muskox on the Kangerlussuaq area graze on graminoids to a larger extent than caribou in the AM region, which has a more diverse diet, including lichen depending on the time of year [17]. This could explain the higher content of hepatic Cu in the muskox, which corroborates the results found by Gamberg et al. [12] that state that caribou from KS have higher hepatic Cu accumulation as a result of dietary graminoids of higher Cu tolerance than lichens. Therefore, it could be suggested that the combined influence of diet and geographical location are major factors affecting these element profiles.
Caribou had significantly higher Cd concentration in the liver, compared to the muskox. In contrast, Cr was detected in the muskox liver and not in the caribou. A similar hepatic accumulation of heavy metals has been reported before in the Canadian Yukon [18], as the local caribou feed heavily on lichen that accumulates heavy metals. The same could apply in this study. However, the concentrations are low, since Greenland has comparatively lower rates of anthropogenic pollution [16]. Nonetheless, edible animal tissues should be monitored for their contaminating elements. Contaminants have been detected in other Arctic mammals such as the hare and ringed seal, derived from pollution coming from Eurasia [19,20,21]. For instance, Johansen et al. [19] has found that liver, kidney and blubber (of marine mammals) of arctic animals have the proclivity to be sources of contaminants, such as mercury (Hg) and Cd, despite ungulate tissues having predominantly very low to medium levels of these elements (non-hazardous levels), according to the authors’ classification.
In the muscle and adipose tissue, Cu was significantly influenced by sex, with females having higher concentrations than males. Our values on the female caribou muscle are within range from those reported in caribou females from AM (8 mg/kg DM) and KS (13 mg/kg DM) regions [12]. Interestingly, there were no significant effects of sex on Cu levels in the hepatic tissue, a main storage site of this element. Copper is an important co-factor for several enzymes such as cytochrome oxidase that participate in oxidation–reduction pathways [22]. It has been previously reported that female muskoxen upregulate these pathways [11]. Ljubojević et al. [23] have found that higher Fe in the liver of female rats is related to metallothionein synthesis and is accompanied by increased malondialdehyde (results from lipid peroxidation) levels, which could be related to increased oxidative stress. In the present study, female ungulates had higher Fe in both muscle and adipose tissue, although without statistical significance in the muscle. By having higher oxidative stress, female ungulates could upregulate oxidation–reduction pathways. The precise reason why these differences have occurred is unclear, and further research could benefit from using a more consistent set of samples (e.g. balanced groups) in order to further explain these discrepancies.
Conclusions and Future Perspectives
The present study provides a glimpse into the element profile of edible tissues of West Greenland caribou and muskoxen, demonstrating differences between the two species. It is apparent that of the two factors “species” and “sex”, the former has the greatest impact on element composition of the three tested tissues, as confirmed in the PCA analysis. This likely reflects species-specific behaviour and physiology. Indeed, future research on these topics and food safety assessments should also include contaminant analyses (e.g. Pb, Cd and As) as different species accumulate different levels of contaminants. It is noteworthy for future studies to also consider, e.g. sampling animals harvested in the same region during the same season. Moreover, studying element concentrations relative to fat-free weight could eliminate diluting effects, since fat content has an influence on tissue element concentration.
References
Cuyler C, Rowell J, Adamczewski J et al (2020) Muskox status, recent variation, and uncertain future. Ambio 49:805–819. https://doi.org/10.1007/s13280-019-01205-x
Cuyler C, Rosing M, Mølgaard H, Heinrich R, Raundrup K (2011; revised 2012) Status of two West Greenland caribou populations 2010; 1) Kangerlussuaq-Sisimiut, 2) Akia-Maniitsoq. Pinngortitaleriffik – Greenland Institute of Natural Resources. Technical Report No. 78. 158 pp. (Part I: 1–86; Part II: 87–158)
Bliss LC, Courtin GM, Pattie DL, Riewe RR, Whitfield DWA, Widden P (1973) Arctic tundra ecosystems. Annu Rev Ecol Syst 4:359–399. https://doi.org/10.1146/annurev.es.04.110173.002043
Klein DR (1992) Comparative ecological and behavioral adaptations of Ovibos moschatus and Rangifer tarandus. Rangifer 12(2):47–55. https://doi.org/10.7557/2.12.2.1016
Ribeiro DM, Mourato MP, Almeida AM (2019) Assessing mineral status in edible tissues of domestic and game animals: a review with a special emphasis in tropical regions. Trop Anim Health Prod 51:1019–1032. https://doi.org/10.1007/s11250-019-01848-8
Lérias JR, Kilminster T, Scanlon T et al (2016) The fat-tail of Damara sheep: an assessment of mineral content as influenced by weight loss. Anim Prod Sci 56:1492. https://doi.org/10.1071/AN14852
Ribeiro DM, Scanlon T, Kilminster T, et al (2020) Mineral profiling of muscle and hepatic tissues of Australian Merino, Damara and Dorper lambs: effect of weight loss. J Anim Physiol Anim Nutr (Berl) 1–8. https://doi.org/10.1111/jpn.13339
Borch-Iohnsen B, Nilssen KJ, Norheim G (1996) Influence of season and diet on liver and kidney content of essential elements and heavy metals in Svalbard reindeer. Biol Trace Elem Res 51:235–247. https://doi.org/10.1007/BF02784078
Blakley BR, Kutz SJ, Tedesco SC, Flood PF (2000) Trace mineral and vitamin concentrations in the liver and serum of wild muskoxen from Victoria Island. J Wildl Dis 36:301–307. https://doi.org/10.7589/0090-3558-36.2.301
Alves SP, Raundrup K, Cabo  et al (2015) Fatty acid composition of muscle, adipose tissue and liver from muskoxen (Ovibos moschatus) living in West Greenland. PLoS One 10:1–21. https://doi.org/10.1371/journal.pone.0145241
Ribeiro DM, Planchon S, Leclercq CC et al (2019) The muscular, hepatic and adipose tissues proteomes in muskox (Ovibosmoschatus): differences between males and females. J Proteomics 208:103480. https://doi.org/10.1016/j.jprot.2019.103480
Gamberg M, Cuyler C, Wang X (2016) Contaminants in two West Greenland caribou populations. Sci Total Environ 554–555:329–336. https://doi.org/10.1016/j.scitotenv.2016.02.154
Staaland H, Olesen CR (1992) Muskox and caribou adaptation to grazing on the Angujaartorfiup Nunaa range in West Greenland. Rangifer 12:105. https://doi.org/10.7557/2.12.2.1027
Nellemann C (1997) Grazing strategies of muskoxen (Ovibos moschatus) during winter in Angujaartorfiup Nunaa in western Greenland. Can J Zool 75:1129–1134. https://doi.org/10.1139/z97-135
Chase LA, Studier EH, Thorisson S (1994) Aspects of nitrogen and mineral nutrition in Icelandic reindeer, Rangifer tarandus. Comp Biochem Physiol Part A Physiol 109:63–73. https://doi.org/10.1016/0300-9629(94)90312-3
Aastrup P, Riget F, Dietz R, Asmund G (2000) Lead, zinc, cadmium, mercury, selenium and copper in Greenland caribou and reindeer (Rangifer tarandus). Sci Total Environ 245:149–159. https://doi.org/10.1016/S0048-9697(99)00440-4
Raundrup K (2018) Movement patterns and habitat selection - insights from West Greenland caribou. PhD Thesis, Aarhus University, pp 113
Gamberg M, Scheuhammer AM (1994) Cadmium in caribou and muskoxen from the Canadian Yukon and Northwest territories. Sci Total Environ 143:221–234. https://doi.org/10.1016/0048-9697(94)90459-6
Johansen P, Muir D, Asmund G, Rigét F (2004) Contaminants in the traditional Greenland diet. Denmark, Copenhagen
Andersen SM (2005) Vitamins and minerals in the traditional Greenland diet. National Environmental Research Institute, Denmark. 44 pp – NERI Technical Report no. 528. http://technical-reports.dmu.dk
Deutch B, Dyerberg J, Pedersen HS et al (2007) Traditional and modern Greenlandic food - dietary composition, nutrients and contaminants. Sci Total Environ 384:106–119. https://doi.org/10.1016/j.scitotenv.2007.05.042
Kincaid RL (2000) Assessment of trace mineral status of ruminants: a review. J Anim Sci 77:1. https://doi.org/10.2527/jas2000.77E-Suppl1x
Ljubojević M, Orct T, Micek V et al (2019) Sex-dependent expression of metallothioneins MT1 and MT2 and concentrations of trace elements in rat liver and kidney tissues: effect of gonadectomy. J Trace Elem Med Biol 53:98–108. https://doi.org/10.1016/j.jtemb.2019.02.010
Acknowledgements
The authors thank the kind collaboration of local hunters who have provided the samples.
Funding
Open access funding provided by FCT|FCCN (b-on). Authors DMR, MPM and AMA acknowledge funding through FCT — Fundação para a Ciência e a Tecnologia, I.P., under the project UIDB/04129/2020 of LEAF-Linking Landscape, Environment, Agriculture and Food, Research Unit and a PhD scholarship to DMR (ref. SFRH/BD/143992/2019).
Author information
Authors and Affiliations
Contributions
Material preparation, data collection and analysis were performed by David Miguel Ribeiro, Miguel Pedro Mourato and Katrine Raundrup. André Martinho de Almeida was responsible for the study conception and team management. The draft of the manuscript was written by David Miguel Ribeiro and André Martinho de Almeida. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The samples used in this study were obtained during the annual hunting season in Greenland. The animals were hunted for consumption and not for research purposes. As such, no ethics committee permission was required to conduct this research.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ribeiro, D.M., Raundrup, K., Mourato, M.P. et al. The Effect of Species and Sex on the Element Content of Muskox (Ovibos moschatus) and Caribou (Rangifer tarandus groenlandicus) Tissues. Biol Trace Elem Res 201, 4718–4725 (2023). https://doi.org/10.1007/s12011-023-03562-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03562-x