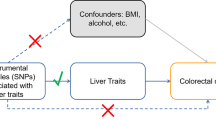
Abstract
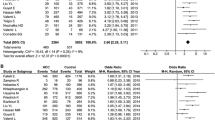
The association between circulating copper and the risk of liver cancer has been investigated by previous studies, while the findings were inconsistent. Thus, we aimed to evaluate the association between circulating copper and liver cancer by using meta-analysis and Mendelian randomization (MR). For meta-analysis, PubMed and Web of Science were searched to identify eligible studies published before April 4, 2022. Standardized mean difference (SMD) with 95% confidence interval (CI) in circulating copper level between liver cancer patients and controls were pooled. Furthermore, we selected genetic instruments for circulating copper from a genome-wide association study (GWAS) to conduct MR analysis. The summary statistics related to liver cancer were obtained from two large independent cohorts, UKBB and FinnGen, respectively. MR analysis was performed mainly by inverse-variance weighted (IVW) approach, followed by maximum-likelihood method as sensitivity analysis. In meta-analysis of eight studies, circulating copper was found to be higher in liver cancer patients (SMD: 1.65; 95% CI: 0.65 to 2.65) with high heterogeneity (I2 = 96.40%, P = 0.001). However, inconsistent findings were observed among subgroups with high evidence. In MR analysis, genetically predicted circulating copper was not significantly associated with the risk of liver cancer by IVW in UKBB (OR: 1.38; 95% CI: 0.72 to 2.65) and FinnGen (OR: 1.10; 95% CI: 0.69 to 1.73) separately, and the pooled results produced similar results (OR: 1.18, 95% CI: 0.81 to 1.72). Moreover, non-significant finding was confirmed by using maximum-likelihood method. There is no sufficient evidence to demonstrate that high levels of circulating copper increase the risks of liver cancer.



Similar content being viewed by others
Data Availability
All data described in the manuscript and codes would be available upon request pending.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Aleksandrova K, Boeing H, Nothlings U, Jenab M, Fedirko V, Kaaks R, Lukanova A, Trichopoulou A, Trichopoulos D, Boffetta P, Trepo E, Westhpal S, Duarte-Salles T, Stepien M, Overvad K, Tjonneland A, Halkjaer J, Boutron-Ruault MC, Dossus L, Racine A, Lagiou P, Bamia C, Benetou V, Agnoli C, Palli D, Panico S, Tumino R, Vineis P, Bueno-de-Mesquita B, Peeters PH, Gram IT, Lund E, Weiderpass E, Quiros JR, Agudo A, Sanchez MJ, Gavrila D, Barricarte A, Dorronsoro M, Ohlsson B, Lindkvist B, Johansson A, Sund M, Khaw KT, Wareham N, Travis RC, Riboli E, Pischon T (2014) Inflammatory and metabolic biomarkers and risk of liver and biliary tract cancer. Hepatol 60:858–871. https://doi.org/10.1002/hep.27016
Gurusamy K (2007) Trace element concentration in primary liver cancers–a systematic review. Biol Trace Elem Res 118:191–206. https://doi.org/10.1007/s12011-007-0008-x
Thomas MB, Zhu AX (2005) Hepatocellular carcinoma: the need for progress. J Clin Oncol 23:2892–2899. https://doi.org/10.1200/JCO.2005.03.196
Tashiro H, Kawamoto T, Okubo T, Koide O (2003) Variation in the distribution of trace elements in hepatoma. Biol Trace Elem Res 95:49–63. https://doi.org/10.1385/BTER:95:1:49
Mandishona E, MacPhail AP, Gordeuk VR, Kedda MA, Paterson AC, Rouault TA, Kew MC (1998) Dietary iron overload as a risk factor for hepatocellular carcinoma in Black Africans. Hepatol 27:1563–1566. https://doi.org/10.1002/hep.510270614
Chen XB, Wei YH, Chen XK, Zhong J, Zou YB, Nie JY (2019) Manganese levels and hepatocellular carcinoma: a systematic review and meta-analysis based on Asian cohort. Med (Baltimore) 98:e16748. https://doi.org/10.1097/MD.0000000000016748
Shenkin A (2006) The key role of micronutrients. Clin Nutr 25:1–13. https://doi.org/10.1016/j.clnu.2005.11.006
Maeda T, Shimada M, Harimoto N, Tsujita E, Maehara S, Rikimaru T, Tanaka S, Shirabe K, Maehara Y (2005) Role of tissue trace elements in liver cancers and non-cancerous liver parenchyma. Hepatogastroenterol 52:187–190
Liaw KY, Lee PH, Wu FC, Tsai JS, Lin-Shiau SY (1997) Zinc, copper, and superoxide dismutase in hepatocellular carcinoma. Am J Gastroenterol 92:2260–2263
Fang AP, Chen PY, Wang XY, Liu ZY, Zhang DM, Luo Y, Liao GC, Long JA, Zhong RH, Zhou ZG, Xu YJ, Xu XJ, Ling WH, Chen MS, Zhang YJ, Zhu HL (2019) Serum copper and zinc levels at diagnosis and hepatocellular carcinoma survival in the Guangdong Liver Cancer Cohort. Int J Cancer 144:2823–2832. https://doi.org/10.1002/ijc.31991
Poo JL, Rosas-Romero R, Montemayor AC, Isoard F, Uribe M (2003) Diagnostic value of the copper/zinc ratio in hepatocellular carcinoma: a case control study. J Gastroenterol 38:45–51. https://doi.org/10.1007/s005350300005
Stepien M, Hughes DJ, Hybsier S, Bamia C, Tjonneland A, Overvad K, Affret A, His M, Boutron-Ruault MC, Katzke V, Kuhn T, Aleksandrova K, Trichopoulou A, Lagiou P, Orfanos P, Palli D, Sieri S, Tumino R, Ricceri F, Panico S, Bueno-de-Mesquita HB, Peeters PH, Weiderpass E, Lasheras C, BonetBonet C, Molina-Portillo E, Dorronsoro M, Huerta JM, Barricarte A, Ohlsson B, Sjoberg K, Werner M, Shungin D, Wareham N, Khaw KT, Travis RC, Freisling H, Cross AJ, Schomburg L, Jenab M (2017) Circulating copper and zinc levels and risk of hepatobiliary cancers in Europeans. Br J Cancer 116:688–696. https://doi.org/10.1038/bjc.2017.1
Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G (2008) Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 27:1133–1163. https://doi.org/10.1002/sim.3034
Zeng Z, Zhang W, Qian Y, Huang H, Wu DJH, He Z, Ye D, Mao Y, Wen C (2020) Association of telomere length with risk of rheumatoid arthritis: a meta-analysis and Mendelian randomization. Rheumatol (Oxford) 59:940–947. https://doi.org/10.1093/rheumatology/kez524
Sekula P, Del Greco MF, Pattaro C, Kottgen A (2016) Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol 27:3253–3265. https://doi.org/10.1681/ASN.2016010098
Hartwig FP, Borges MC, Horta BL, Bowden J, Davey Smith G (2017) Inflammatory biomarkers and risk of schizophrenia: a 2-sample Mendelian randomization study. JAMA Psychiat 74:1226–1233. https://doi.org/10.1001/jamapsychiatry.2017.3191
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605. https://doi.org/10.1007/s10654-010-9491-z
Islam MA, Khandker SS, Alam SS, Kotyla P, Hassan R (2019) Vitamin D status in patients with systemic lupus erythematosus (SLE): a systematic review and meta-analysis. Autoimmun Rev 18:102392. https://doi.org/10.1016/j.autrev.2019.102392
Chinn S (2000) A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med 19:3127–3131. https://doi.org/10.1002/1097-0258(20001130)19:22%3c3127::aid-sim784%3e3.0.co;2-m
Yue H, Shan L, Bin L (2018) The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: a systematic review and meta-analysis. Gastric Cancer 21:579–587. https://doi.org/10.1007/s10120-018-0812-3
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634. https://doi.org/10.1136/bmj.315.7109.629
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Si S, Li J, Tewara MA, Xue F (2021) Genetically determined chronic low-grade inflammation and hundreds of health outcomes in the UK Biobank and the FinnGen population: a phenome-wide Mendelian randomization study. Front Immunol 12:720876. https://doi.org/10.3389/fimmu.2021.720876
Yuan S, Carter P, Vithayathil M, Kar S, Giovannucci E, Mason AM, Burgess S, Larsson SC (2020) Iron status and cancer risk in UK Biobank: a two-sample Mendelian randomization study. Nutrients 12. https://doi.org/10.3390/nu12020526
Evans DM, Zhu G, Dy V, Heath AC, Madden PA, Kemp JP, McMahon G, St Pourcain B, Timpson NJ, Golding J, Lawlor DA, Steer C, Montgomery GW, Martin NG, Smith GD, Whitfield JB (2013) Genome-wide association study identifies loci affecting blood copper, selenium and zinc. Hum Mol Genet 22:3998–4006. https://doi.org/10.1093/hmg/ddt239
Park JH, Wacholder S, Gail MH, Peters U, Jacobs KB, Chanock SJ, Chatterjee N (2010) Estimation of effect size distribution from genome-wide association studies and implications for future discoveries. Nat Genet 42:570–575. https://doi.org/10.1038/ng.610
Palmer TM, Lawlor DA, Harbord RM, Sheehan NA, Tobias JH, Timpson NJ, Davey Smith G, Sterne JA (2012) Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res 21:223–242. https://doi.org/10.1177/0962280210394459
Wu F, Huang Y, Hu J, Shao Z (2020) Mendelian randomization study of inflammatory bowel disease and bone mineral density. BMC Med 18:312. https://doi.org/10.1186/s12916-020-01778-5
Zhou J, Liu C, Sun Y, Francis M, Ryu MS, Grider A, Ye K (2021) Genetically predicted circulating levels of copper and zinc are associated with osteoarthritis but not with rheumatoid arthritis. Osteoarthritis Cartilage 29:1029–1035. https://doi.org/10.1016/j.joca.2021.02.564
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J (2017) A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med 36:1783–1802. https://doi.org/10.1002/sim.7221
Bowden J, Davey Smith G, Haycock PC, Burgess S (2016) Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 40:304–314. https://doi.org/10.1002/gepi.21965
Elzeiny MA, Elzefzafy WM, Shahin RS, Atef N, Ahmed MG (2010) Serum levels of selenium, zinc, copper and iron in patients with post viral hepatitis liver cirrhosis& hepatocellular carcinoma. ASIAN ACAD MANAG J 8:1
Nayak SB, Yashwanth S, Pinto SM, Bhat VR, Mayya SS (2005) Serum copper, ceruloplasmin, protein thiols and thiobarbituric acid reactive substance status in liver cancer associated with elevated levels of alpha-fetoprotein. Indian J Physiol Pharmacol 49:341–344
Lin CC, Huang JF, Tsai LY, Huang YL (2006) Selenium, iron, copper, and zinc levels and copper-to-zinc ratios in serum of patients at different stages of viral hepatic diseases. Biol Trace Elem Res 109:15–24. https://doi.org/10.1385/BTER:109:1:015
Nagasue N, Kolno H, Chang YC, Nakamura T (1989) Iron, copper and zinc levels in serum and cirrhotic liver of patients with and without hepatocellular carcinoma. Oncol 46:293–296. https://doi.org/10.1159/000226735
Porcu C, Antonucci L, Barbaro B, Illi B, Nasi S, Martini M, Licata A, Miele L, Grieco A, Balsano C (2018) Copper/MYC/CTR1 interplay: a dangerous relationship in hepatocellular carcinoma. Oncotarget 9:9325–9343. https://doi.org/10.18632/oncotarget.24282
Fu L, Xie H, Huang J, Chen L (2020) Rapid determination of trace elements in serum of hepatocellular carcinoma patients by inductively coupled plasma tandem mass spectrometry. Anal Chim Acta 1112:1–7. https://doi.org/10.1016/j.aca.2020.03.054
Shanbhag VC, Gudekar N, Jasmer K, Papageorgiou C, Singh K, Petris MJ (2021) Copper metabolism as a unique vulnerability in cancer. Biochim Biophys Acta Mol Cell Res 1868:118893. https://doi.org/10.1016/j.bbamcr.2020.118893
Osredkar J, Sustar N (2011) Copper and zinc, biological role and significance of copper/zinc imbalance. J Clinic Toxicol S3:001. https://doi.org/10.4172/2161-0495.S3-001
Gurusamy K, Davidson BR (2007) Trace element concentration in metastatic liver disease: a systematic review. J Trace Elem Med Biol 21:169–177. https://doi.org/10.1016/j.jtemb.2007.03.003
Lin L, Yan L, Liu Y, Qu C, Ni J, Li H (2020) The burden and trends of primary liver cancer caused by specific etiologies from 1990 to 2017 at the global, regional, national, age, and sex level results from the Global Burden of Disease Study 2017. Liver Cancer 9:563–582. https://doi.org/10.1159/000508568
Verduijn M, Siegerink B, Jager KJ, Zoccali C, Dekker FW (2010) Mendelian randomization: use of genetics to enable causal inference in observational studies. Nephrol Dial Transplant 25:1394–1398. https://doi.org/10.1093/ndt/gfq098
Song J, Liu K, Chen W, Liu B, Yang H, Lv L, Sun X, Mao Y, Ye D (2021) Circulating vitamin D levels and risk of vitiligo: evidence from meta-analysis and two-sample Mendelian randomization. Front Nutr 8:782270. https://doi.org/10.3389/fnut.2021.782270
He B, Shi J, Wang X, Jiang H, Zhu HJ (2020) Genome-wide pQTL analysis of protein expression regulatory networks in the human liver. BMC Biol 18:97. https://doi.org/10.1186/s12915-020-00830-3
Chen MH, Raffield LM, Mousas A, Sakaue S, Huffman JE, Moscati A, Trivedi B, Jiang T, Akbari P, Vuckovic D, Bao EL, Zhong X, Manansala R, Laplante V, Chen M, Lo KS, Qian H, Lareau CA, Beaudoin M, Hunt KA, Akiyama M, Bartz TM, Ben-Shlomo Y, Beswick A, Bork-Jensen J, Bottinger EP, Brody JA, van Rooij FJA, Chitrala K, Cho K, Choquet H, Correa A, Danesh J, Di Angelantonio E, Dimou N, Ding J, Elliott P, Esko T, Evans MK, Floyd JS, Broer L, Grarup N, Guo MH, Greinacher A, Haessler J, Hansen T, Howson JMM, Huang QQ, Huang W, Jorgenson E, Kacprowski T, Kahonen M, Kamatani Y, Kanai M, Karthikeyan S, Koskeridis F, Lange LA, Lehtimaki T, Lerch MM, Linneberg A, Liu Y, Lyytikainen LP, Manichaikul A, Martin HC, Matsuda K, Mohlke KL, Mononen N, Murakami Y, Nadkarni GN, Nauck M, Nikus K, Ouwehand WH, Pankratz N, Pedersen O, Preuss M, Psaty BM, Raitakari OT, Roberts DJ, Rich SS, Rodriguez BAT, Rosen JD, Rotter JI, Schubert P, Spracklen CN, Surendran P, Tang H, Tardif JC, Trembath RC, Ghanbari M, Volker U, Volzke H, Watkins NA, Zonderman AB, Program VAMV, Wilson PWF, Li Y, Butterworth AS, Gauchat JF, Chiang CWK, Li B, Loos RJF, Astle WJ, Evangelou E, van Heel DA, Sankaran VG, Okada Y, Soranzo N, Johnson AD, Reiner AP, Auer PL, Lettre G (2020) Trans-ethnic and ancestry-specific blood-cell genetics in 746,667 individuals from 5 global populations. Cell 182:1198-1213e1114. https://doi.org/10.1016/j.cell.2020.06.045
Acknowledgements
The authors sincerely thank the researchers and participants of the observational articles included in meta-analysis and the researchers of GWAS study for their collection and sharing of large-scale data.
Funding
This work was supported by grants from the Natural Science Foundation of Zhejiang Province (Nos. LQ20H260008 and LQ21H260001) and the Zhejiang Chinese Medical University Foundation (2020ZG01, 2020ZG16).
Author information
Authors and Affiliations
Contributions
DY and ST L conceived and designed the study. WW C, DJ, and KL conducted data analysis and interpreted the results. WW C and ZW Z drafted the manuscript, XH S, YY M, ST L, and DY revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Because the current study was based on public data and published articles, no additional ethical approval was required.
Consent to Participate
The written informed consent of all participants was obtained by the respective Institutional Review Boards.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, W., Zhang, Z., Liu, K. et al. Circulating Copper and Liver Cancer. Biol Trace Elem Res 201, 4649–4656 (2023). https://doi.org/10.1007/s12011-023-03554-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03554-x