Abstract
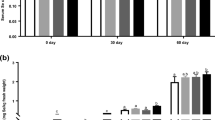
This study was aimed at investigating the effects of diet iron levels on the blood iron status, tissue iron content, mRNA levels, and the activity of iron-containing enzymes in different tissues of squabs. A total of 120 pairs of healthy Silver Feather King parental pigeons with similar average body weight and egg production were randomly divided into 5 groups with 8 replicates and 3 pairs of pigeons per replicate. The five groups of breeding pigeons were fed an iron-unsupplemented basal diet and basal diet supplemented with 75, 150, 300, and 600 mg iron/kg, respectively. The diets were fed in the form of granular feed based on corn, soybean meal, wheat, and sorghum. A broken line model was used for regression analysis. The results showed that plasma iron (PI), serum ferritin, iron contents in crop milk and liver, liver catalase (CAT) activity, and heart succinate dehydrogenase (SDH) activity were affected by iron levels (P < 0.05). And PI, serum ferritin, iron content in crop milk, and heart SDH activity increased quadratically (P < 0.05), but the iron content and CAT activity in the liver decreased quadratically (P < 0.005) as dietary iron level increased. According to the broken-line model of serum ferritin fitting (P < 0.002), the optimal dietary iron level of breeding pigeons was estimated to be 193 mg/kg. In conclusion, serum ferritin is a sensitive index to evaluate the iron requirement of the breeding pigeon with two squabs, and the recommended iron supplemental level is 193 mg/kg.
Similar content being viewed by others
Data Availability
The data of this study will be made available on reasonable request.
References
Ji F, Zhang D, Shao Y, Yu X, Liu X, Shan D, Wang Z (2020) Changes in the diversity and composition of gut microbiota in pigeon squabs infected with Trichomonas Gallinae. Sci Rep 10(1):19978. https://doi.org/10.1038/s41598-020-76821-9
Xu Q, Li H, Zhou W, Zou X, Dong X (2020) Age-related changes in serum lipid levels, hepatic morphology, antioxidant status, lipid metabolism related gene expression and enzyme activities of domestic pigeon squabs (Columba Livia). Animals (Basel) 10(7):1121. https://doi.org/10.3390/ani10071121
Xie W, Fu Z, Pan N, Yan H, Wang X, Gao C (2019) Leucine promotes the growth of squabs by increasing crop milk protein synthesis through the tor signaling pathway in the domestic pigeon (Columba Livia). Poult Sci 98(11):5514–5524. https://doi.org/10.3382/ps/pez296
Chen M, Fu Z, Jiang S, Wang X, Yan H, Gao C (2020) Targeted disruption of TORC1 retards young squab growth by inhibiting the synthesis of crop milk protein in breeding pigeon (Columba Livia). Poult Sci 99(1):416–422. https://doi.org/10.3382/ps/pez513
Shao Y, Ma W, Ji F, Sun X, Du S, Li X, Li Q, Wang Z (2021) Exploration of proteomics analysis of crop milk in pigeons (Columba livia) during the lactation period. ACS Omega 6(42):27726–27736. https://doi.org/10.1021/acsomega.1c02977
Xu Q, Wen J, Wang X, Zou X, Dong X (2021) Maternal dietary linoleic acid altered intestinal barrier function in domestic pigeons (Columba livia). Br J Nutr 126(7):1003–1016. https://doi.org/10.1017/S0007114520004973
Ji F, Zhang S, An Y, Wang Z, Shao Y, Du S, Li X, Sun X (2022) Influence of dietary phosphorus concentrations on the performance of rearing pigeons (Columba livia), and bone properties of squabs. Poult Sci 101(4):101744. https://doi.org/10.1016/j.psj.2022.101744
Chang L, Zhang R, Fu S, Mu C, Tang Q, Bu Z (2019) Effects of different dietary calcium levels on the performance, egg quality, and albumen transparency of laying pigeons. Animals (Basel) 9(3):110. https://doi.org/10.3390/ani9030110
Qin Z, Caruso J, Lai B, Matusch A, Becker J (2011) Trace metal imaging with high spatial resolution: applications in biomedicine. Metallomics 3(1):28–37. https://doi.org/10.1039/c0mt00048e
Wang W, Di X, D’agostino R, Torti S, Torti F (2007) Excess capacity of the iron regulatory protein system. J Biol Chem 282(34):24650–24659. https://doi.org/10.1074/jbc.M703167200
Taschetto D, Vieira S, Angel C, Stefanello C, Kindlein L, Ebbing M, Simões C (2017) Iron requirements of broiler breeder hens. Poult Sci 96(11):3920–3927. https://doi.org/10.3382/ps/pex208
Tan Z, Lu P, Adewole D, Diarra MS, Gong J, Yang C (2020) Iron requirement in the infection of Salmonella and its relevance to poultry health. J Appl Poult Res 30(1):100101. https://doi.org/10.1016/j.japr.2020.09.016
Baker R, Greer F (2010) Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 Years of Age). Pediatrics 126(5):1040–1050. https://doi.org/10.1542/peds.2010-2576
Zhang B, Sui F, Wang B, Wang Y, Li W (2020) Dietary combined supplementation of iron and Bacillus subtilis enhances reproductive performance, eggshell quality, nutrient digestibility, antioxidant capacity, and hematopoietic function in breeder geese. Poult Sci 99(11):6119–6127. https://doi.org/10.1016/j.psj.2020.06.077
National Research Council (1994) Nutrient requirements for poultry, 9th rev. edn. National Academies Press, Washington, D.C. https://doi.org/10.17226/2114
Yousefi A, Saki A (2019) Iron loaded chitooligosaccharide nanoparticles reduces incidence of bacterial chondronecrosis with osteomyelitis in broiler chickens. Iran J Appl Anim Sci 9(2):329–336
Behroozlak MA, Daneshyar M, Farhoomand P, Nikoo A (2019) Effect of replacing dietary FeSO4 with FeHPO4 nanoparticles on growth performance, carcass characteristics and tissue iron content in broilers. Iran J Appl Anim Sci 9(4):709–716
Hagler L, Askew E, Neville J, Mellick P, Coppes R, Lowder JF (1981) Influence of dietary iron deficiency on hemoglobin, myoglobin, their respective reductases, and skeletal muscle mitochondrial respiration. Am J Clin Nutr 34(10):2169–2177. https://doi.org/10.1093/ajcn/34.10.2169
Siimes M, Refino C, Dallman P (1980) Manifestation of iron deficiency at various levels of dietary iron intake. Am J Clin Nutr 33(3):570–574. https://doi.org/10.1093/ajcn/33.3.570
Liao X, Ma C, Lu L, Zhang L, Luo X (2017) Determination of dietary iron requirements by full expression of iron-containing cytochrome c oxidase in the heart of broilers from 22 to 42 d of age. Br J Nutr 118(7):493–499. https://doi.org/10.1017/S0007114517002458
de Deungria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK (2000) Perinatal iron deficiency decreases cytochrome c oxidase (cytox) activity in selected regions of neonatal rat brain. Pediatr Res 48(2):169–76. https://doi.org/10.1203/00006450-200008000-00009
Feng J, Ma W, Xu Z, He J, Wang Y, Liu J (2008) The effect of iron glycine chelate on tissue mineral levels, fecal mineral concentration, and liver antioxidant enzyme activity in weanling pigs. Anim Feed Sci Technol 150(1):106–113. https://doi.org/10.1016/j.anifeedsci.2008.07.004
Ma X, Liao X, Lu L, Li S, Zhang L, Luo X (2016) Determination of dietary iron requirements by full expression of iron-containing enzymes in various tissues of broilers. J Nutr 146(11):2267–2273. https://doi.org/10.3945/jn.116.237750
Zhao D, Li X, Wang Z, Shi Z, Qin S, Shao Y, Sun X, Zhang X (2022) Effects of different levels of iron in breeding pigeon diet on body weight, slaughter performance, meat quality and blood indexes of pigeons. Chin J Anim Nutr 34(6):3635–3644
Tran H, Schlageter-Tello A, Caprez A, Miller PS, Hall MB, Weiss WP, Kononoff PJ (2020) Development of feed composition tables using a statistical screening procedure. J Dairy Sci 103(4):3786–3803. https://doi.org/10.3168/jds.2019-16702
Huebers H, Eng M, Josephson B, Ekpoom N, Rettmer R, Labbé R, Pootrakul P, Finch CA (1987) Plasma iron and transferrin iron-binding capacity evaluated by colorimetric and immunoprecipitation methods. Clin Chem 33(2):273–277. https://doi.org/10.1093/clinchem/33.2.273
Anon (1978) The measurement of total and unsaturated iron-binding capacity in serum. Br J Haematol 38(2):281–287. https://doi.org/10.1111/j.1365-2141.1978.tb01044.x
Huang Y, Lu L, Luo X, Liu B (2007) An optimal dietary zinc level of broiler chicks fed a corn-soybean meal diet. Poult Sci 86(12):2582–2589. https://doi.org/10.3382/ps.2007-00088
Livak K, Schmittgen T (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(t)) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Nulton-Persson A, Szweda L (2001) Modulation of mitochondrial function by hydrogen peroxide. J Biol Chem 276(26):23357–23361. https://doi.org/10.1074/jbc.M100320200
Hou H, Li B, Zhao X, Zhuang Y, Ren G, Yan M, Cai Y, Zhang X, Chen L (2009) The effect of pacific cod (Gadus Macrocephalus) skin gelatin polypeptides on UV radiation-induced skin photoaging in ICR mice. Food Chem 115(3):945–950. https://doi.org/10.1016/j.foodchem.2009.01.015
Lu L, Chang B, Liao X, Wang R, Zhang L, Luo X (2016) Use of molecular biomarkers to estimate manganese requirements for broiler chickens from 22 to 42 d of age. Br J Nutr 116(9):1512–1518. https://doi.org/10.1017/S0007114516003640
Robbins KR, Norton HW, Baker DH (1979) Estimation of nutrient requirements from growth data. J Nutr 109(10):1710–4. https://doi.org/10.1093/jn/109.10.1710
Corzo A, Dozier W, Kidd M (2006) Dietary lysine needs of late-developing heavy broilers. Poult Sci 85(3):457–461. https://doi.org/10.1093/ps/85.3.457
Bainton D, Finch C (1964) The diagnosis of iron deficiency anemia. AM J MED 37(1):62–70. https://doi.org/10.1016/0002-9343(64)90212-8
Chen S, Wu X, Wang X, Shao Y, Tu Q, Yang H, Yin J, Yin Y (2020) Responses of intestinal microbiota and immunity to increasing dietary levels of iron using a piglet model. Front Cell Dev Biol 8:603392. https://doi.org/10.3389/fcell.2020.603392
Jaeggi T, Kortman GA, Moretti D, Chassard C, Holding P, Dostal A, Boekhorst J, Timmerman HM, Swinkels DW, Tjalsma H, Njenga J, Mwangi A, Kvalsvig J, Lacroix C, Zimmermann MB (2015) Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 64(5):731–742. https://doi.org/10.1136/gutjnl-2014-307720
Kaitha S, Bashir M, Ali T (2015) Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol 6(3):62–72. https://doi.org/10.4291/wjgp.v6.i3.62
Saneela S, Iqbal R, Raza A, Qamar MF (2019) Hepcidin: a key regulator of iron. J Pak Med Assoc 69(8):1170–1175
Kwiecień M, Samolińska W, Bujanowicz-haraś B (2015) Effects of iron-glycine chelate on growth, carcass characteristic, liver mineral concentrations and haematological and biochemical blood parameters in broilers. J Anim Physiol Anim Nutr (Berl) 99(6):1184–1196. https://doi.org/10.1111/jpn.12322
Kobayashi Y, Imai N, Uenishi K (2020) Attempt to determine the cut-off value of serum ferritin for iron deficiency in male college student runners. J Nutr Sci Vitaminol (Tokyo) 66(5):432–440. https://doi.org/10.3177/jnsv.66.432
Furugouri K (1972) Effect of elevated dietary levels of iron on iron store in liver, some blood constituents and phosphorus deficiency in young swine. J Anim Sci 34(4):573–577. https://doi.org/10.2527/jas1972.344573x
Wensing T, A A, S A, (1986) Some aspects of extra iron supply in veal calf fattening. Vet Res Commun 10(4):283–296. https://doi.org/10.1007/BF02213991
Lin X, Gou Z, Wang Y, Li L, Fan Q, Ding F, Zheng C, Jiang S (2020) Effects of dietary iron level on growth performance, immune organ indices and meat quality in Chinese yellow broilers. Animals (Basel) 10(4):670. https://doi.org/10.3390/ani10040670
Xie C, Elwan H, Elnesr S, Dong X, Feng J, Zou X (2019) Effects of iron glycine chelate on laying performance, antioxidant activities, serum biochemical indices, iron concentrations and transferrin mRNA expression in laying hens. J Anim Physiol Anim Nutr (Berl) 103(2):547–554. https://doi.org/10.1111/jpn.13061
Rincker M, Hill G, Link J, Rowntree J (2004) Effects of dietary iron supplementation on growth performance, hematological status, and whole-body mineral concentrations of nursery pigs. J Anim Sci 82(11):3189–3197. https://doi.org/10.2527/2004.82113189x
Knutson M (2010) Iron-sensing proteins that regulate hepcidin and enteric iron absorption. Annu Rev Nutr 30:149–171. https://doi.org/10.1146/annurev.nutr.012809.104801
Yang L, Wang H, Yang X, Wu Q, An P, Jin X, Liu W, Huang X, Li Y, Yan S, Shen S, Liang T, Min J, Wang F (2020) Auranofin mitigates systemic iron overload and induces ferroptosis via distinct mechanisms. Signal Transduct Target Ther 5(1):138. https://doi.org/10.1038/s41392-020-00253-0
Li S, Lu L, Hao S, Wang Y, Zhang L, Liu S, Liu B, Li K, Luo X (2011) Dietary manganese modulates expression of the manganese-containing superoxide dismutase gene in chickens. J Nutr 141(2):189–194. https://doi.org/10.3945/jn.110.126680
Goncalves J, Moog S, Morin A, Gentric G, Müller S, Morrell AP, Kluckova K, Stewart TJ, Andoniadou CL, Lussey-Lepoutre C, Bénit P, Thakker A, Vettore L, Roberts J, Rodriguez R, Mechta-Grigoriou F, Gimenez-Roqueplo AP, Letouzé E, Tennant DA, Favier J (2021) Loss of SDHB promotes dysregulated iron homeostasis, oxidative stress, and sensitivity to ascorbate. Cancer Res 81(13):3480–3494. https://doi.org/10.1158/0008-5472.CAN-20-2936
Rao J, Jagadeesan V (1996) Lipid Peroxidation and activities of antioxidant enzymes in iron deficiency and effect of carcinogen feeding. Free Radic Biol Med 21(1):103–108. https://doi.org/10.1016/0891-5849(95)02212-0
Tavsan Z, Ayar Kayali H (2013) The Effect of iron and copper as an essential nutrient on mitochondrial electron transport system and lipid peroxidation in Trichoderma harzianum. Appl Biochem Biotechnol 170(7):1665–1675. https://doi.org/10.1007/s12010-013-0273-4
Wan J, Ren H, Wang J (2019) Iron toxicity, lipid peroxidation and ferroptosis after intracerebral haemorrhage. Stroke Vasc Neurol 4(2):93–95. https://doi.org/10.1136/svn-2018-000205
Weiss Sachdev S, Sunde RA (2001) Selenium regulation of transcript abundance and translational efficiency of glutathione peroxidase-1 and -4 in rat liver. Biochem J 357(Pt 3):851–8. https://doi.org/10.1042/0264-6021:3570851
Zhang L, Lu L, Zhang L, Luo X (2016) The chemical characteristics of organic iron sources and their relative bioavailabilities for broilers fed a conventional corn-soybean meal diet. J Anim Sci 94(6):2378–2396. https://doi.org/10.2527/jas.2016-0297
Liu Y, Beyer A, Aebersold R (2016) On the dependency of cellular protein levels on mRNA abundance. Cell 165(3):535–550. https://doi.org/10.1016/j.cell.2016.03.014
Funding
This study was funded by the business fund of Beijing Academy of Agriculture and Forestry Science (under Grant number: XMSSYJJ2021) and national modern agricultural science and technology city industry cultivation and achievements benefiting the people project of Beijing Municipal Commission of Science and Technology (number: Z171100001517003).
Author information
Authors and Affiliations
Contributions
Dongdong Zhao carried out the experiments. Shizhen Qin, Zhaoguo Shi, Xing Li, and Yangyang Wang processed the data. Zheng Wang and Yuxin Shao designed the experiment and wrote the paper. The final manuscript was read and approved by all authors.
Corresponding author
Ethics declarations
Ethics Approval
The study was carried out in accordance with the guidelines set by the Animal Care and Use Committee (permit number: SYXK-2017–0005) of the Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agriculture and Forestry Sciences (IAHVM-BAAFS), Beijing, China. The protocols were approved by the Animal Care and Use Committee of IAHVM-BAAFS.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Zhao, D., Qin, S. et al. Effects of Dietary Supplementation with Iron in Breeding Pigeons on the Blood Iron Status, Tissue Iron Content, and Full Expression of Iron-Containing Enzymes of Squabs. Biol Trace Elem Res 201, 4538–4546 (2023). https://doi.org/10.1007/s12011-022-03530-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03530-x