Abstract
The objective of this study was to determine the relationship of urinary nickel (U-Ni) exposure to serum lipid profiles and the mediation effect of body mass index (BMI) in a US general population. We analyzed the cross-sectional data from 3517 participants in the National Health and Nutrition Examination Survey (NHANES) (2017-March 2020). Multivariable linear regression and restricted cubic spline (RCS) regression were conducted to explore the association of U-Ni with four serum lipids and four lipids-derived indicators. Mediation analysis was performed to examine the effect of BMI on the relationship between U-Ni levels and serum lipid profiles. Compared with the lowest quartile, the β with 95% confidence intervals (CIs) in the highest quartile were − 12.83 (− 19.42, − 6.25) for total cholesterol (TC) (P for trend < 0.001), − 12.76 (− 19.78, − 5.74) for non-high-density lipoprotein cholesterol (non-HDL-C) (P for trend = 0.001) and − 0.29 (− 0.51, − 0.07) for TC/HDL-C (P for trend = 0.007) in the fully adjusted model. RCS plots showed the linear association of log2-transformed U-Ni levels with TC, non-HDL-C and TC/HDL-C (P for nonlinearity = 0.294, 0.152, and 0.087, respectively). Besides, BMI decreased monotonically in correlation with increasing U-Ni levels (P for trend < 0.001). Mediation analysis revealed that BMI significantly mediated the relationship of U-Ni to TC, non-HDL-C and TC/HDL-C with mediated proportions of 11.17%, 22.20% and 36.44%, respectively. In summary, our findings suggest that BMI mediates the negative association of U-Ni with TC, non-HDL-C, and TC/HDL-C in the US general population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dyslipidemia, particularly elevated low-density lipoprotein cholesterol (LDL-C) level, is a major risk factor for cardiovascular disease (CVD), the leading cause of global mortality and disability [1]. The global burden of dyslipidemia has increased dramatically over the past 30 years. Projections estimated that elevated LDL-C level was the 15th leading risk factor for death in 1990, rising to 11th in 2007 and 8th in 2019, and more than one-third of deaths caused by CVD were attributable to elevated LDL-C levels [2]. Therefore, the management of dyslipidemia and lipid profiles is essential in the primary and secondary prevention of CVD [3, 4]. Abundant studies have shown that LDL-C, non-high-density lipoprotein cholesterol (non-HDL-C), and triglycerides (TG) are atherosclerotic lipid profiles and support their causal roles in atherosclerotic CVD (ASCVD) [5]. Among them, the cumulative exposure of an artery to LDL-C over years remains a principal determinant of ASCVD initiation and progression [6]. Besides CVD, elevated lipid profiles and dyslipidemia are also associated with a range of other diseases, including diabetes [7], chronic kidney disease [8], valvular heart disease [9], non-alcoholic fatty liver disease [10], Alzheimer’s disease [11], and coronavirus disease 2019 (COVID-19) [12], posing an enormous threat to human health.
With economic development and accelerated industrialization, humans are easily exposed to environmental metals due to their widespread presence in the electronics, optics, glass, and medical industries [13]. Nickel (Ni), a hard silvery-white metal, is a very abundant natural element. Ni can be combined with other metals, such as iron, copper, chromium, and zinc, to form alloys [14]. These alloys are used to make coins, jewelry, and items such as valves and heat exchangers. Most of Ni is used to make stainless steel [15]. Meanwhile, it is worth noting that coronary stents and occlusion devices used to repair atrial septal defects and patent foramen ovale usually contain nitinol, an alloy containing Ni [16, 17]. The main routes of human exposure to environmental Ni include ingestion of contaminated food, inhalation of ambient air and tobacco, and skin contact [15]. Ni has been recognized as an essential nutrient for some microorganisms, plants, and animal species. However, no existing evidence denotes its nutritional value in humans [18]. Therefore, it is necessary to explore the effects of Ni exposure on human health.
Previous studies have demonstrated that the most common adverse health effect of Ni is an allergic reaction and the prevalence of Ni allergy in the general population is approximately 8% to 19% [19]. In addition, exposure to Ni can cause chronic bronchitis, lung fibrosis, and even lung and sinus cancer, and Ni is classified as a group 2B carcinogen (possibly carcinogenic) [15]. Ni exposure is also associated with an increased risk of congenital heart defects in offspring [20]. However, it remains largely unclear whether Ni exposure influences human lipid levels. Moreover, several studies have revealed that Ni exposure may be associated with lower body weight or body mass index (BMI) [21,22,23,24], and BMI, as a surrogate indicator of body weight and obesity, is recognized to be closely related to lipid profiles and dyslipidemia. However, whether BMI plays a mediating role in this relationship remains unknown.
Based on the existing evidence from previous studies, the association between Ni exposure and blood lipids remains largely unclear and the mediating role of BMI in this association needs to be further elucidated. Using the representative and large-scale US population-based National Health and Nutrition Examination Survey (NHANES 2017-March 2020) data, the present study aimed to investigate the mediation role of BMI on the association between urinary Ni (U-Ni) level, a commonly used and long-term biomarker of individual Ni exposure [25], and serum lipid profiles in the general population.
Materials and Methods
Study Design and Participants
NHANES is a major program of the National Center for Health Statistics (NCHS) and is designed to assess the health status of the US general population. NHANES uses a complex statistical process to reflect the civilian non-institutionalized resident population information [26]. The survey design, methods, population, and data are publicly available from the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm). NHANES protocols were approved by the NCHS Research Ethics Review Board and all participants provided written informed consent.
For the present analysis, we consolidated data from NHANES (2017-March 2020) because U-Ni was only measured during this time period. NHANES suspended field operations in March 2020 due to the COVID-19 pandemic. Therefore, data from 2019 to March 2020 were combined with data from the 2017–2018 cycle to form a nationally representative sample. Among the 15,560 participants in the total sample, 4569 participants had complete data on U-Ni. Participants with missing data on TC (N = 1052), HDL-C (N = 1052), LDL-C (N = 3057), and TG (N = 3057) were further excluded, respectively. Ultimately, a total of 3517 participants for TC and HDL-C and 1512 participants for LDL-C and TG were retained and enrolled for analysis (Online Resource 1, Supplementary Fig. 1).
Measurement of U-Ni
To measure U-Ni, a casual (or spot) urine specimen was collected from the participants after confirmation of no background contamination. U-Ni levels were detected using inductively coupled plasma mass spectrometry (ICP-MS) after a simple dilution sample preparation step [27]. Liquid samples were reduced to small droplets in an argon aerosol via a nebulizer, and then, the droplets entered the ICP where the thermal energy atomized the sample and then ionized the atoms. The ions were pulled into the mass spectrometer where they were focused through the universal cell technology (UCT), the quadrupole mass filter, and finally were selectively counted in rapid sequence at the detector according to their mass-to-charge (m/z) ratio. When detecting U-Ni, the UCT was filled with helium gas and operated in a kinetic energy discrimination (KED) mode to eliminate polyatomic interferences. The detailed detection method for U-Ni is available online at https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/P_UNI.htm. The lower limit of detection (LLOD) for U-Ni was 0.31 µg/L. According to NHANES Laboratory Method File, when the detected U-Ni level was lower than LLOD, the detected value was expressed by LLOD divided by the square root of 2 (i.e., 0.22 µg/L).
Assessment of Covariates
Demographic information in this study included age, gender, race, education level and the ratio of family income to poverty (PIR) and was obtained through uniform interviews. Medical history information, including hypertension, diabetes, CVD and lipid-lowering drug use, as well as lifestyle information, including smoking status, alcohol intake and physical activity, was ascertained by standardized questionnaires. Laboratory results, including high-sensitivity C-reactive protein (hsCRP) and urinary creatinine (UCr), were determined in NHANES mobile examination centers.
Race was categorized into non-Hispanic white, non-Hispanic black, Mexican American and others. Educational level was classified into three categories: less than high school, high school or equivalent and college or above according to NHANES demographic files. Individuals who smoked < 100 cigarettes in their entire life were defined as never smokers; those who had smoked at least 100 cigarettes but did not smoke at the time of survey were considered former smokers; and those who had smoked at least 100 cigarettes and still smoked cigarettes at the time of survey were considered current smokers. Participants were considered alcohol users in NHANES 2017-March 2020 if they had had at least 1 drink of any kind of alcohol, which differed from previous NHANES questionnaires. Physical activity for each participant was categorized as follows: (1) below, less than 600 MET min/week or 150 min/week of moderate-intensity exercise; (2) meet, 600 to 1200 MET min/week or 150 to 300 min/week of moderate-intensity exercise; or (3) exceed, at least 1200 MET min/week or 300 min/week of moderate-intensity exercise [26]. Hypertension was defined as the systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg, self-reported history of hypertension or taking anti-hypertensive agents [28]. Diabetes was defined as a fasting plasma glucose ≥ 126 mg/dL, a random plasma glucose ≥ 200 mg/dL, glycated hemoglobin A1c ≥ 6.5%, self-reported history of diabetes or taking anti-hyperglycemic agents [29]. CVD was ascertained using a standardized questionnaire and included a composite event of congestive heart failure, coronary heart disease, angina pectoris, heart attack and stroke described previously [30]. Laboratory results, including hsCRP and UCr, were determined using standardized NHANES detection methods. BMI is the weight divided by the square of height (kg/m2). The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease-Epidemiology Collaboration equation [31].
Ascertainment of Serum Lipid Profiles
In the present study, TC was measured using an enzymatic assay, in which esterified cholesterol was converted to cholesterol by cholesterol esterase. The resulting cholesterol was then acted upon by cholesterol oxidase to produce cholest-4-en-3-one and hydrogen peroxide. The hydrogen peroxide then reacted with 4-aminophenazone in the presence of peroxidase to produce a colored product measured at 505 nm. HDL-C was measured using a magnesium/dextran sulfate solution that was first added to the specimen to form water-soluble complexes with non-HDL cholesterol fractions. With the addition of reagent 2, HDL-C esters were converted to HDL-C by PEG-cholesterol esterase. The HDL-C was acted upon by PEG-cholesterol oxidase, and the hydrogen peroxide produced from this reaction combined with 4-amino-antipyrine and HSDA under the action of peroxidase to form a purple/blue pigment measured photometrically at 600 nm. TG was measured using a lipoprotein lipase from microorganisms for the rapid and complete hydrolysis of TG to glycerol, followed by oxidation to dihydroxyacetone phosphate and hydrogen peroxide. The hydrogen peroxide then reacted with 4-aminophenazone and 4-chlorophenol to form a red dyestuff and its color intensity was directly proportional to TG concentration and could be measured photometrically. The Friedewald formula was used to compute LDL-C levels ([LDL-C] = [TC] − [HDL-C] − [TG/5]) [32]. Detailed laboratory methods for lipid profiles can be accessed at https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Laboratory&Cycle=2017-2020. In addition, we also calculated four lipids-derived indicators: (1) non-HDL-C equals TC minus HDL-C; (2) TC/HDL-C equals TC divided by HDL-C; (3) LDL-C/HDL-C equals LDL-C divided by HDL-C; (4) remnant cholesterol (RC) equals TC minus HDL-C minus LDL-C [33].
Statistical Analysis
Given that NHANES aims to produce representative data of the non-institutionalized US general population, sample weights, clustering, and strata are recommended for statistical analysis to account for the unequal probability of selection, oversampling of certain subpopulations, and non-response adjustment [26]. Statistical analyses in the present study took into account the complex sampling design of NHANES and were weighted. Descriptive statistics for continuous variables were expressed in the form of weighted median (25th percentile, 75th percentile) and categorical variables were represented by weighted percentages (standard error [SE]). SE was obtained using the Taylor series linearization.
U-Ni values were categorized into quartiles, and the lowest quartile (Q1) served as the reference group. Since U-Ni had a positively skewed distribution, log2-transformation was also performed to improve the normality of the data [34]. A multivariable linear regression model was used to calculate β and corresponding 95% confidence intervals (CIs). No adjustment was made in model 1. Model 2 was adjusted for age, gender and race. Model 3 was the same as model 2 with additional adjustments for education level, PIR, hypertension, diabetes, smoking, alcohol use, physical activity, CVD, lipid-lowering drug use, hsCRP, eGFR and UCr. Model 4 (fully adjusted model) was adjusted for model 3 plus BMI (both continuous and categorical [< 25, 25–29.9, or ≥ 30 kg/m2] variables) to further adjust for the possible influence of BMI with reference to published methods [35, 36]. We conducted linear regression analysis twice using U-Ni as continuous (log2-transformed) and categorical (quartiles) variables respectively. To further investigate the dose–response relationship between U-Ni levels and serum lipid profiles, restricted cubic splines (RCS) with 3 knots at 10th, 50th and 90th percentiles were performed.
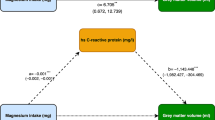
Mediation analysis with 1000 bootstrap resamples and adjustment for covariates was performed to evaluate whether BMI mediated the relationship between U-Ni and serum lipid profiles using the “mediation” package in R software to estimate the indirect effect, direct effect, and total effect [37]. The mediated proportion was calculated as indirect effect/(indirect effect + direct effect) × 100%. Regression coefficients correspond to distinct pathways that characterize (1) the total effect of U-Ni on serum lipid profiles (path C); (2) the direct effect of U-Ni on serum lipid profiles when BMI (mediator) is included in the model (path C′); (3) the effect of U-Ni on BMI (path A); (4) the effect of BMI on serum lipid profiles (path B); and (5) the indirect effect of BMI on the association between U-Ni and serum lipid profiles (path A*B) (Fig. 1).
Schematic diagram of the mediation effect analysis. Path C indicates the total effect; path C′ indicates the direct effect. The indirect effect is estimated as the multiplication of paths A and B (path A*B). The mediated proportion is calculated as indirect effect/(indirect effect + direct effect) × 100%
Several sensitivity analyses were performed in the study. Firstly, to correct for differences in urine dilution in spot urine samples, U-Ni levels were corrected by UCr (μg/g UCr) [38]. Secondly, since this study included both adults (the vast majority) and minors, to determine whether the findings were valid in adults, we excluded participants < 20 years old and re-run the statistical analysis [30]. Thirdly, to explore the relationship between U-Ni and overall blood lipids, a multivariable logistic regression was performed to assess the association between U-Ni and dyslipidemia, a dichotomous outcome [39]. Fourthly, previous experimental studies showed that the effect of Ni on plasma and liver lipids was influenced by iron [40]. Thus, serum iron levels were added for further adjustment.
All statistical analyses were performed using Stata version 16.0 (StataCorp LLC, College Station, TX, USA) and R version 4.1.0. A P value < 0.05 (two-tailed) was considered statistically significant.
Results
Baseline Characteristics of the Study Participants
The study included 3517 participants, 49.6% of whom were males. The weighted median age (25th percentile, 75th percentile) of the study participants was 42 (25, 59) years. The participants were predominantly non-Hispanic White (60.4%) and educated above the high school level (60.0%). The prevalence of hypertension was 33.6% and the prevalence of diabetes was 12.5%. The proportion of lipid-lowering drug use was 24.8%. The median BMI of the participants was 27.6 (23.4, 32.6) kg/m2. Detailed baseline information in this study is summarized in Table 1.
Association between U-Ni and Serum Lipid Profiles
Multiple linear regression was conducted to analyze the association of U-Ni, in both continuous and categorical variables form, with serum lipid profiles and is shown in Table 2 and Supplementary Table 1. The continuous analysis demonstrated that log2-transformed U-Ni was significantly and negatively correlated with TC, non-HDL-C and TC/HDL-C (all P < 0.05 in Model 3 and Model 4). Using the lowest quartile (Q1) of U-Ni as the reference, the β with 95% CI in the highest quartile (Q4) were − 12.83 (− 19.42, − 6.25) for total cholesterol (TC) (P for trend < 0.001), − 12.76 − 19.78, − 5.74) for non-HDL-C (P for trend = 0.001) and − 0.29 (− 0.51, − 0.07) for TC/HDL-C (P for trend = 0.007) in the fully adjusted model (model 4) and remained consistent in model 3 (all P for trend < 0.001). The Q4 of U-Ni was also associated with lower LDL-C and LDL-C/HDL-C levels compared with Q1 (β − 9.80; 95% CI (− 19.41, − 0.20), P = 0.046 and β − 0.26; 95% CI (− 0.44, − 0.07), P = 0.009, respectively) in model 3, but not in model 4. No significant association between U-Ni and HDL-C, TG and RC was observed (Table 2). The results did not change when BMI was included as a categorical variable (< 25, 25–29.9, or ≥ 30 kg/m2) (Online Resource 1, Supplementary Table 1).
In addition, RCS plots were consistent with the above results and showed that the association of log2-transformed U-Ni levels with TC, non-HDL-C and TC/HDL-C was linear (P for nonlinearity = 0.294, 0.152 and 0.087, respectively) (Fig. 2).
Restricted cubic spline (RCS) plot of the association between log2-transformed U-Ni levels and eight serum lipid profiles. RCS regression was adjusted for age, gender, race, education level, PIR, hypertension, diabetes, smoking, alcohol use, physical activity, cardiovascular disease, lipid-lowering drug use, hsCRP, eGFR, UCr and BMI (model 4). The solid lines and shadow bands represent the corresponding lipid profiles values and 95% confidence intervals
Association between U-Ni and BMI
The association between U-Ni and BMI (mediator) with adjustment for covariates was assessed and presented in Table 3. The continuous analysis showed that log2-transformed U-Ni had a significant negative association with BMI (β − 0.80; 95% CI (− 1.13, − 0.47), P < 0.001). As the quartiles of U-Ni increased, BMI monotonically decreased (P for trend < 0.001).
Association between BMI and Serum Lipid Profiles
A significant correlation was observed between BMI (mediator) and serum lipid profiles, as shown in Table 4. In the multivariable linear regression models, an elevated BMI was associated with a significantly elevated TC, non-HDL-C, TC/HDL-C, LDL-C, LDL-C/HDL-C, TG and RC level (all β > 0 and P ≤ 0.001), as well as a significantly decreased HDL-C level (β − 0.67; 95% CI (− 0.77, − 0.57), P < 0.001).
Mediation Effects of BMI on the Association between U-Ni and Lipid Profiles
The mediation effect analysis shown in Table 5 revealed that BMI significantly mediated the relationship of U-Ni to TC, non-HDL-C and TC/HDL-C and the mediated proportions were 11.17%, 22.20% and 36.44% (P = 0.018, < 0.001 and < 0.001), respectively. Besides, the indirect and total effects of BMI on the association between log2-transformed U-Ni and HDL-C were significant (P < 0.001 and P = 0.002), while the direct effect was not significant (P = 0.648), indicating that BMI played a complete mediation role and the mediated proportion was 95.58% (P = 0.002). BMI did not significantly mediate the association of U-Ni with LDL-C, LDL-C/HDL-C, TG and RC (all P > 0.05).
Sensitivity Analyses
The first sensitivity analysis revealed UCr-corrected U-Ni was still significantly and negatively associated with TC, non-HDL-C and TC/HDL-C, as well as LDL-C and LDL-C/HDL-C (all P for trend < 0.05 in model 3 or model 4) (Online Resource 1, Supplementary Table 2). Moreover, this association of U-Ni with TC, non-HDL-C and TC/HDL-C was also solid in adults (P for trend = 0.002, 0.002 and 0.017, respectively, model 4) (Supplementary Table 3). U-Ni was also negatively associated with the prevalence of dyslipidemia (P for trend = 0.014 in model 3 and 0.042 in model 4) (Supplementary Table 4). Supplementary Table 5 reveals that this association between U-Ni and serum lipid profiles remained true after further adjusting for iron. All sensitivity analyses were substantially unchanged and consistent with the main analyses.
Discussion
In the population-based cross-sectional study of a US nationally representative sample, we investigated the mediation effect of BMI, a frequently used anthropometric indicator, on the association between U-Ni and multiple serum lipid profiles. Based on multiple statistical models and sensitivity analyses, our findings demonstrated that U-Ni was linearly and negatively associated with TC, non-HDL-C and TC/HDL-C in the US general population and the association was robust. BMI may act as an essential mediator in this association.
Given the increasing exposure to environmental metals and the growing burden of dyslipidemia, elucidating the relationship between environmental metals and blood lipids or dyslipidemia is warranted. Previous epidemiological studies have underscored that several metals, such as lead, mercury and cadmium, are associated with dyslipidemia risk [41, 42]. Nevertheless, studies concerning the effect of Ni on blood lipids are still scarce. Ni, as a transition metal and one of the ferromagnetic elements, may be an essential trace element for mammals. However, although Ni is present at a concentration of approximately 0.5 nM in the human bloodstream, neither the source of Ni requirement nor a single Ni-dependent enzyme has been detected in mammals [43]. Beyond the risk of allergy, lung dysfunction and lung cancer, the effects of Ni exposure on human health and the mechanisms underlying these effects are not well understood.
Regarding the effects of Ni on blood lipids, the results of previous experimental studies are inconsistent. An experiment conducted on rats showed that Ni sulfate treatment caused a significant increase in serum LDL-C and TG levels [44]. Similarly, Cloez and colleagues found that low concentrations of Ni increased lipid synthesis in mice [45]. However, another study declared that feeding rats with Ni acetate reduced serum cholesterol levels, showing anti-cholesterol properties [46]. Ni chloride was also observed to decrease serum total lipids, phospholipids and bilirubin in rats [47]. The levels of cholesterol were found to decrease after Ni sulfate exposure [48]. A microbiological study also indicated that Ni exposure resulted in a decrease in infrared reflectance bands associated with fatty acids and lipids, suggesting that Ni might downregulate lipid synthesis [49]. In addition to the controversial findings, most of these studies were animal studies. In general, extrapolation from experimental results in animals to humans is difficult.
Data on Ni exposure in humans are minimal. In a study of Chinese occupational workers, higher levels of Ni exposure were associated with an increased risk of diabetes [50]. Ni exposure was also associated with decreased diastolic blood pressure [25]. In a study of 88 healthy students, higher concentrations of Ni in PM2.5 components were associated with higher HDL-C [51], which was consistent with our first sensitivity analysis (model 3). However, that study had a small sample size and did not focus on other blood lipid profiles.
The biological mechanisms underlying the relationship of Ni exposure to lipids are currently unidentified. Zhang et al. identified 21 differential metabolites associated with bile acids, lipid and phospholipid metabolism pathways after Ni exposure, indicating that bile acid metabolism dysfunction might play a role in this process [52]. Besides, Ni chloride was linked to an increase in the erythrocyte activity of superoxide dismutase, an antioxidant metalloenzyme that catalyzes the dismutation of superoxide anion free radicals to generate oxygen and hydrogen peroxide and plays a crucial role in the balance between oxidation and antioxidation [47]. In addition, Ni could inhibit the essential sulfhydryl groups in enzymes associated with fatty acid synthesis and acylation, and the sulfhydryl groups are indispensable for lipid synthesis [53]. Consistent with the literature, we demonstrated a significant correlation between U-Ni and lipid profiles.
Our results also revealed that an increase in U-Ni was associated with a decrease in BMI. Previous studies also found a negative correlation between U-Ni and weight, which might be due to increased apoptosis and glutathione depletion [24]. Similar results were observed in a study of pregnant women which showed that prenatal Ni exposure was associated with lower preterm birth weight [23]. An occupational study also revealed that Ni-exposed workers had a lower BMI than controls (23.20 ± 0.63 in workers vs. 24.63 ± 0.76 in controls) [22]. Besides, another study demonstrated that compared with non-workers, brick kiln workers had higher Ni levels but lower rate of overweight and obesity (29.4% in workers vs. 73.0% in non-workers) [21]. Our study also found a negative correlation between U-Ni and BMI, consistent with these previous studies. BMI is a recognized risk factor for dyslipidemia, which was also consistent with our findings showing that BMI was positively associated with atherosclerotic lipids and negatively associated with HDL-C. However, there was no report on the contribution of BMI-mediated U-Ni to lipid profiles and we proposed a mediator role of BMI for the first time.
This study has several strengths. We used nationally representative data from NHANES, enabling us to generalize our findings to a broader population. Moreover, the multiple outcomes (eight lipid profiles), statistical models and sensitivity analyses ensured the robustness of the results. Nevertheless, several limitations should also be addressed. Firstly, NHANES used casual (or spot) urine specimens to detect U-Ni concentrations, without considering the effect of Ni exposure duration on lipid profiles. Secondly, although a series of confounders were adjusted, potential confounders might still not be included in the statistical models, as in other epidemiological studies. Thirdly, given the cross-sectional design of NHANES, a causal relationship between U-Ni and serum lipid profiles cannot be confirmed. Moreover, the precise mechanisms of the effects of Ni on lipids have not been fully elucidated. Therefore, the association shown in this study needs to be confirmed in future prospective studies.
Conclusions
Our findings suggest that U-Ni is negatively associated with TC, non-HDL-C and TC/HDL-C and the association is linear in the US general population. BMI is identified for the first time as an important mediator in the observed relationship between U-Ni exposure and serum lipid profiles. Further research is warranted to validate our findings and to explain the potential mechanisms.
Data Availability
The datasets used and/or analyzed during the current study are available from the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm).
Abbreviations
- ASCVD:
-
Atherosclerotic cardiovascular disease
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- COVID-19:
-
Coronavirus disease 2019
- CVD:
-
Cardiovascular disease
- eGFR:
-
Estimated glomerular filtration rate
- HDL-C:
-
High-density lipoprotein cholesterol
- hsCRP:
-
High-sensitivity C-reactive protein
- ICP-MS:
-
Inductively coupled plasma mass spectrometry
- LDL-C:
-
Low-density lipoprotein cholesterol
- LLOD:
-
Lower limit of detection
- NHANES:
-
National Health and Nutrition Examination Survey
- non-HDL-C:
-
Non-high-density lipoprotein cholesterol
- PIR:
-
The ratio of family income to poverty
- RC:
-
Remnant cholesterol
- RCS:
-
Restricted cubic spline
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- UCr:
-
Urinary creatinine
- U-Ni:
-
Urinary nickel
References
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM et al (2020) Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J Am Coll Cardiol 76(25):2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010
Pirillo A, Casula M, Olmastroni E, Norata GD, Catapano AL (2021) Global epidemiology of dyslipidaemias. Nat Rev Cardiol 18(10):689–700. https://doi.org/10.1038/s41569-021-00541-4
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ et al (2019) 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 140(11):e596–e646. https://doi.org/10.1161/CIR.0000000000000678
Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H et al (2018) 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39(2):119–177. https://doi.org/10.1093/eurheartj/ehx393
Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS et al (2019) Atherosclerosis. Nat Rev Dis Primers 5(1):56. https://doi.org/10.1038/s41572-019-0106-z
Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E et al (2017) Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 38(32):2459–2472. https://doi.org/10.1093/eurheartj/ehx144
Kane JP, Pullinger CR, Goldfine ID, Malloy MJ (2021) Dyslipidemia and diabetes mellitus: Role of lipoprotein species and interrelated pathways of lipid metabolism in diabetes mellitus. Curr Opin Pharmacol 61:21–27. https://doi.org/10.1016/j.coph.2021.08.013
Hager MR, Narla AD, Tannock LR (2017) Dyslipidemia in patients with chronic kidney disease. Rev Endocr Metab Disord 18(1):29–40. https://doi.org/10.1007/s11154-016-9402-z
Mata P, Alonso R, Perez de Isla L, Badimon L (2021) Dyslipidemia and aortic valve disease. Curr Opin Lipidol 32(6):349–354. https://doi.org/10.1097/MOL.0000000000000794
Katsiki N, Mikhailidis DP, Mantzoros CS (2016) Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism 65(8):1109–1123. https://doi.org/10.1016/j.metabol.2016.05.003
Reitz C (2013) Dyslipidemia and the risk of Alzheimer’s disease. Curr Atheroscler Rep 15(3):307. https://doi.org/10.1007/s11883-012-0307-3
Liu Y, Pan Y, Yin Y, Chen W, Li X (2021) Association of dyslipidemia with the severity and mortality of coronavirus disease 2019 (COVID-19): a meta-analysis. Virol J 18(1):157. https://doi.org/10.1186/s12985-021-01604-1
Li Z, Feng X, Li G, Bi X, Zhu J, Qin H et al (2013) Distributions, sources and pollution status of 17 trace metal/metalloids in the street dust of a heavily industrialized city of central China. Environ Pollut 182:408–416. https://doi.org/10.1016/j.envpol.2013.07.041
Ahlstrom MG, Thyssen JP, Wennervaldt M, Menne T, Johansen JD (2019) Nickel allergy and allergic contact dermatitis: A clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermatitis 81(4):227–241. https://doi.org/10.1111/cod.13327
Genchi G, Carocci A, Lauria G, Sinicropi MS, Catalano A (2020) Nickel: Human Health and Environmental Toxicology. Int J Environ Res Public Health 17(3) https://doi.org/10.3390/ijerph17030679.
Bahrani B, Moghaddam N, DeKoven J (2019) Cross-sectional Survey of Nickel Allergy Management in the Context of Intracardiac Device Implantation. Dermatitis 30(3):213–221. https://doi.org/10.1097/DER.0000000000000466
Nagaraja S, Sullivan SJL, Stafford PR, Lucas AD, Malkin E (2018) Impact of nitinol stent surface processing on in-vivo nickel release and biological response. Acta Biomater 72:424–433. https://doi.org/10.1016/j.actbio.2018.03.036
Song X, Fiati Kenston SS, Kong L, Zhao J (2017) Molecular mechanisms of nickel induced neurotoxicity and chemoprevention. Toxicology 392:47–54. https://doi.org/10.1016/j.tox.2017.10.006
Ahlstrom MG, Thyssen JP, Menne T, Johansen JD (2017) Prevalence of nickel allergy in Europe following the EU Nickel Directive - a review. Contact Dermatitis 77(4):193–200. https://doi.org/10.1111/cod.12846
Zhang N, Chen M, Li J, Deng Y, Li SL, Guo YX et al (2019) Metal nickel exposure increase the risk of congenital heart defects occurrence in offspring: A case-control study in China. Medicine (Baltimore) 98(18):e15352. https://doi.org/10.1097/MD.0000000000015352
David M, Jahan S, Hussain J, Rehman H, Cloete KJ, Afsar T et al (2022) Biochemical and reproductive biomarker analysis to study the consequences of heavy metal burden on health profile of male brick kiln workers. Sci Rep 12(1):7172. https://doi.org/10.1038/s41598-022-11304-7
David M, Qurat Ul A, Afzal M, Shoaib M, Aman F, Cloete KJ et al (2020) Study of occupational exposure to brick kiln emissions on heavy metal burden, biochemical profile, cortisol level and reproductive health risks among female workers at Rawat. Pakistan Environ Sci Pollut Res Int 27(35):44073–44088. https://doi.org/10.1007/s11356-020-10275-4
Sun X, Jiang Y, Xia W, Jin S, Liu W, Lin X et al (2018) Association between prenatal nickel exposure and preterm low birth weight: possible effect of selenium. Environ Sci Pollut Res Int 25(26):25888–25895. https://doi.org/10.1007/s11356-018-2622-x
Zheng GH, Liu CM, Sun JM, Feng ZJ, Cheng C (2014) Nickel-induced oxidative stress and apoptosis in Carassius auratus liver by JNK pathway. Aquat Toxicol 147:105–111. https://doi.org/10.1016/j.aquatox.2013.12.015
Liu Y, Wu M, Xu B, Kang L (2022) Association between the urinary nickel and the diastolic blood pressure in general population. Chemosphere 286(Pt 3):131900. https://doi.org/10.1016/j.chemosphere.2021.131900
Bao W, Liu B, Rong S, Dai SY, Trasande L, Lehmler HJ (2020) Association Between Bisphenol A Exposure and Risk of All-Cause and Cause-Specific Mortality in US Adults. JAMA Netw Open 3(8):e2011620. https://doi.org/10.1001/jamanetworkopen.2020.11620
Jones DR, Jarrett JM, Stukes D, Baer A, McMichael M, Wallon K et al (2021) Development and validation of a biomonitoring method to measure As, Cr, and Ni in human urine samples by ICP-UCT-MS. Int J Hyg Environ Health 234:113713. https://doi.org/10.1016/j.ijheh.2021.113713
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M et al (2018) 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 39(33):3021–3104. https://doi.org/10.1093/eurheartj/ehy339
Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R et al (2022) 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 45(Supplement_1):S17–S38. https://doi.org/10.2337/dc22-S002
Zhang Y, Huang M, Zhuang P, Jiao J, Chen X, Wang J et al (2018) Exposure to acrylamide and the risk of cardiovascular diseases in the National Health and Nutrition Examination Survey 2003–2006. Environ Int 117:154–163. https://doi.org/10.1016/j.envint.2018.04.047
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006
Mehta R, Reyes-Rodriguez E, Yaxmehen Bello-Chavolla O, Guerrero-Diaz AC, Vargas-Vazquez A, Cruz-Bautista I et al (2018) Performance of LDL-C calculated with Martin’s formula compared to the Friedewald equation in familial combined hyperlipidemia. Atherosclerosis 277:204–210. https://doi.org/10.1016/j.atherosclerosis.2018.06.868
Sandesara PB, Virani SS, Fazio S, Shapiro MD (2019) The Forgotten Lipids: Triglycerides, Remnant Cholesterol, and Atherosclerotic Cardiovascular Disease Risk. Endocr Rev 40(2):537–557. https://doi.org/10.1210/er.2018-00184
Bland JM, Altman DG (1996) The use of transformation when comparing two means. BMJ 312(7039):1153. https://doi.org/10.1136/bmj.312.7039.1153
Dong X, Li Y, Yang K, Zhang L, Xue Y, Yu S et al (2020) Mediation effect of body mass index on the association between spicy food intake and hyperuricemia in rural Chinese adults: the Henan rural cohort study. BMC Public Health 20(1):1629. https://doi.org/10.1186/s12889-020-09736-9
Park S, Choi NK (2019) The relationships of blood lead level, body mass index, and osteoarthritis in postmenopausal women. Maturitas 125:85–90. https://doi.org/10.1016/j.maturitas.2019.04.215
Valente MJ, Rijnhart JJM, Smyth HL, Muniz FB, MacKinnon DP (2020) Causal Mediation Programs in R, Mplus, SAS, SPSS, and Stata. Struct Equ Modeling 27(6):975–984. https://doi.org/10.1080/10705511.2020.1777133
Mendy A, Gasana J, Vieira ER (2012) Urinary heavy metals and associated medical conditions in the US adult population. Int J Environ Health Res 22(2):105–118. https://doi.org/10.1080/09603123.2011.605877
Zhou J, Cai L, Ni S, Zhong Z, Yang M, Yu H et al (2021) Associations of dietary PUFA with dyslipidaemia among the US adults: the findings from National Health and Nutrition Examination Survey (NHANES) 2009-2016. Br J Nutr. 1-9. https://doi.org/10.1017/S0007114521002300
Nielsen FH (1980) Effect of form of iron on nickel deprivation in the rat: Plasma and liver lipids. Biol Trace Elem Res 2(3):199–210. https://doi.org/10.1007/BF02785355
Kang P, Shin HY, Kim KY (2021) Association between Dyslipidemia and Mercury Exposure in Adults. Int J Environ Res Public Health 18(2) https://doi.org/10.3390/ijerph18020775.
Zhu X, Fan Y, Sheng J, Gu L, Tao Q, Huang R et al (2021) Association Between Blood Heavy Metal Concentrations and Dyslipidemia in the Elderly. Biol Trace Elem Res 199(4):1280–1290. https://doi.org/10.1007/s12011-020-02270-0
Ragsdale SW (2009) Nickel-based Enzyme Systems. J Biol Chem 284(28):18571–18575. https://doi.org/10.1074/jbc.R900020200
Das Gupta A, Das SN, Dhundasi SA, Das KK (2008) Effect of garlic (Allium sativum) on heavy metal (nickel II and chromium VI) induced alteration of serum lipid profile in male albino rats. Int J Environ Res Public Health 5(3):147–151. https://doi.org/10.3390/ijerph2008050020
Cloez I, Dumont O, Piciotti M, Bourre JM (1987) Alterations of lipid synthesis in the normal and dysmyelinating trembler mouse sciatic nerve by heavy metals (Hg, Pb, Mn, Cu, Ni). Toxicology 46(1):65–71. https://doi.org/10.1016/0300-483x(87)90138-7
Schroeder HA (1968) Serum cholesterol levels in rats fed thirteen trace elements. J Nutr 94(4):475–480. https://doi.org/10.1093/jn/94.4.475
Novelli EL, Rodrigues NL, Nagahashi AM, Sforcin JM, Ribas BO (1990) Nickel chloride effects on erythrocyte generation of superoxide radical. Braz J Med Biol Res 23(8):643–645. https://www.ncbi.nlm.nih.gov/pubmed/2101086
Rehman K, Fatima F, Waheed I, Akash MSH (2018) Prevalence of exposure of heavy metals and their impact on health consequences. J Cell Biochem 119(1):157–184. https://doi.org/10.1002/jcb.26234
Gupta AD, Karthikeyan S (2016) Individual and combined toxic effect of nickel and chromium on biochemical constituents in E. coli using FTIR spectroscopy and Principle component analysis. Ecotoxicol Environ Saf 130:289–294. https://doi.org/10.1016/j.ecoenv.2016.04.025
Yang AM, Cheng N, Pu HQ, Liu SM, Li JS, Bassig BA et al (2015) Metal Exposure and Risk of Diabetes and Prediabetes among Chinese Occupational Workers. Biomed Environ Sci 28(12):875–883. https://doi.org/10.3967/bes2015.121
He ZZ, Guo PY, Xu SL, Zhou Y, Jalaludin B, Leskinen A et al (2021) Associations of Particulate Matter Sizes and Chemical Constituents with Blood Lipids: A Panel Study in Guangzhou. China Environ Sci Technol 55(8):5065–5075. https://doi.org/10.1021/acs.est.0c06974
Zhang Q, Chang X, Wang X, Zhan H, Gao Q, Yang M et al (2021) A metabolomic-based study on disturbance of bile acids metabolism induced by intratracheal instillation of nickel oxide nanoparticles in rats. Toxicol Res (Camb) 10(3):579–591. https://doi.org/10.1093/toxres/tfab039
Khan AA, Malhotra SS (1987) Effects of vanadium, nickel and sulphur dioxide on polar lipid biosynthesis in jack pine. Phytochemistry 26(6):1627–1630. https://doi.org/10.1016/S0031-9422(00)82258-X
Acknowledgements
The authors would like to thank all the team staff and participants in NHANES for providing the publicly available data.
Author information
Authors and Affiliations
Contributions
S. Wang: conceptualization, methodology, software, writing—original draft, visualization. T. Shan: conceptualization, methodology, writing—review and editing, visualization. J. Zhu: writing—review and editing, data curation, validation. Q. Jiang: software, writing—review and editing. L. Gu: software, writing—review and editing. J. Sun: writing—review and editing, data curation. Y. Bao: writing—review and editing, data curation. B. Deng: data curation. H. Wang: writing—review and editing. L. Wang: conceptualization, project administration, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Ethics Approval and Consent To Participate
The NHANES 2017–2020 was approved by the NCHS Research Ethics Review Board (Continuation of Protocol #2011–17 and Protocol #2018-01), and each participant signed the written informed consent.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, S., Shan, T., Zhu, J. et al. Mediation Effect of Body Mass Index on the Association of Urinary Nickel Exposure with Serum Lipid Profiles. Biol Trace Elem Res 201, 2733–2743 (2023). https://doi.org/10.1007/s12011-022-03375-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03375-4