Abstract
Copper (Cu) is an essential and important trace element for some significant life processes for most organisms. However, an excessive amount of Cu can be highly toxic. The present study was conducted to elucidate the oxidative stress–induced alteration in transcriptional level of autophagy-related genes in the liver and kidney tissue of fish Channa punctatus after treatment with three different sublethal concentrations of CuSO4 for 28 days. All the studied enzymatic and non-enzymatic oxidative stress markers viz. superoxide dismutase-SOD, catalase-CAT, glutathione peroxidase-GPx, glutathione reductase-GR, and glutathione-GSH showed an increase in their activity levels in the treated groups in a dose-dependent manner. Particularly SOD and CAT have shown a significant hike in activity levels. ROS levels in blood cells increased significantly (p < 0.05) in all the treated groups, i.e., Group II-1/20th of 96 h-LC50 (0.2 mg/L), Group III-1/10th of 96 h-LC50 (0.4 mg/L), and Group IV-1/5 h of 96 h-LC50 (0.8 mg/L) of Cu2+ in a dose-dependent manner as compared to control (Group I). The upregulation in mRNA levels of autophagy-related genes Microtubule-associated protein 1 light chain 3 (LC3), Gamma-aminobutyric acid receptor-associated protein precursor (Gabarap), and Golgi-associated ATPase enhancer of 16 kDa (GATE16), autophagy-related 5 (ATG5) was observed while mammalian target of rapamycin (mTOR) showed downregulation in the liver and kidney tissue of fish. The decrease in mTOR and increase in ATG5 gene expression projects autophagic vesicle formation due to oxidative stress. There was significant induction in micronuclei (MN) frequency in all the treated groups. The highest frequency of MN induced by Cu2+ was recorded in Group IV after 28 days of the exposure period. Thus, it can be concluded that the available information about Cu2+-induced oxidative stress–mediated autophagy in the liver and kidney of fish C. punctatus remains largely unclear to date, so to fill the aforesaid gap, the present study was undertaken, which gives an insight for the mechanisms of autophagy induced by Cu2+ in fish.
Graphical Abstract





Similar content being viewed by others
Data Availability
All the data generated during the study is given in the manuscript.
References
Irwin RJ, Van Mouwerik M, Stevens L, Seese MD, Basham W (1997) Environmental Contaminants Encyclopedia. National Park Service, Water Resources Division, Fort Collins, Colorado. Distributed within the Federal Government as an Electronic Document.
Adams MS, Dillon CT, Vogt S et al (2016) Copper uptake, intracellular localization, and speciation in marine microalgae measured by synchrotron radiation X-ray fluorescence and absorption microspectroscopy. Environ Sci Technol 50:8827–8839. https://doi.org/10.1021/ACS.EST.6B00861
Festa RA, Thiele DJ (2011) Copper: an essential metal in biology. Curr Biol 21:R877. https://doi.org/10.1016/J.CUB.2011.09.040
Yruela I (2005) Copper in plants. Braz J Plant Physiol 17:145–156. https://doi.org/10.1590/S1677-04202005000100012
De Boeck G, Meeus W, De CW, Blust R (2004) Tissue-specific Cu bioaccumulation patterns and differences in sensitivity to waterborne Cu in three freshwater fish: rainbow trout (Oncorhynchus mykiss), common carp (Cyprinus carpio), and gibel carp (Carassius auratus gibelio). Aquat Toxicol 70:179–188. https://doi.org/10.1016/J.AQUATOX.2004.07.001
Deshmukh S, Marathe V (1980) Size-related toxicity of copper & mercury to Lebistes reticulatus (Peter), Labeo rohita (Ham.) & Cyprinus carpio Linn. Indian J Exp Biol 18:421–423
Kaur A, Kaur K (1996) Relative susceptibility of different life stages of Channa punctatus and Cyprinus carpio to nickel-chrome electroplating effluent. Bull Environ Contam Toxicol 575(57):836–841. https://doi.org/10.1007/S001289900265
Oruc HH (2010) Fungicides and their effects on animals. In: Carisse O (ed.) Fungicides, In-Tech Publishers, London, U.K, pp 349–362. https://doi.org/10.5772/555
Eyckmans M, Celis N, Horemans N et al (2011) Exposure to waterborne copper reveals differences in oxidative stress response in three freshwater fish species. Aquat Toxicol 103:112–120. https://doi.org/10.1016/J.AQUATOX.2011.02.010
Grosell MH, Hogstrand C, Wood CM (1997) Cu uptake and turnover in both Cu-acclimated and non-acclimated rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 4:257–276. https://doi.org/10.1016/S0166-445X(96)00843-0
Grosell M, Wood CM (2002) Copper uptake across rainbow trout gills: mechanisms of apical entry. J Exp Biol 205:1179–1188. https://doi.org/10.1242/JEB.205.8.1179
Lauren DJ, McDonald DG (2011) Acclimation to copper by rainbow trout, Salmo gairdneri: biochemistry. Can J Feries Aquat Sci 44:105–111. https://doi.org/10.1139/F87-013
Malhotra N, Ger TR, Uapipatanakul B et al (2020) Review of copper and copper nanoparticle toxicity in fish. Nanomaterials 10:1–28. https://doi.org/10.3390/nano10061126
Rajeshkumar S, Li X (2018) Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicol Rep 5:288. https://doi.org/10.1016/J.TOXREP.2018.01.007
Thounaojam TC, Panda P, Mazumdar P et al (2012) Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol Biochem 53:33–39. https://doi.org/10.1016/J.PLAPHY.2012.01.006
Scherz-Shouval R, Elazar Z (2011) Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci 36:30–38. https://doi.org/10.1016/J.TIBS.2010.07.007
Scherz-Shouval R, Shvets E, Fass E et al (2007) Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J 26:1749–1760. https://doi.org/10.1038/SJ.EMBOJ.7601623
Lee J, Giordano S, Zhang J (2012) Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J 441:523–540. https://doi.org/10.1042/BJ20111451
Palacio-Betancur I, Palacio-Baena JA, Camargo-Guerrero M (2009) Micronuclei test application to wild tropical ichthyic species common in two lentic environments of the low zones in Colombia. Actual Biol 31:67–77
Rubinsztein DC, Ravikumar B, Acevedo-Arozena A et al (2005) Dyneins, autophagy, aggregation and neurodegeneration. Autophagy 1:177–178. https://doi.org/10.4161/AUTO.1.3.2050
Avagliano L, Danti L, Doi P et al (2013) Autophagy in placentas from acidotic newborns: an immunohistochemical study of LC3 expression. Placenta 34:1091–1094. https://doi.org/10.1016/J.PLACENTA.2013.09.004
Karim MR, Kawanago H, Kadowaki M (2014) A quick signal of starvation induced autophagy: transcription versus post-translational modification of LC3. Anal Biochem 465:28–34. https://doi.org/10.1016/J.AB.2014.07.007
Chen D, Zhang Z, Yao H et al (2015) Effects of atrazine and chlorpyrifos on oxidative stress-induced autophagy in the immune organs of common carp (Cyprinus carpio L.). Fish Shellfish Immunol 44:12–20. https://doi.org/10.1016/J.FSI.2015.01.014
Awasthi Y, Ratn A, Prasad R et al (2019) A protective study of curcumin associated with Cr6+ induced oxidative stress, genetic damage, transcription of genes related to apoptosis and histopathology of fish, Channa punctatus (Bloch, 1793). Environ Toxicol Pharmacol 71:1–10. https://doi.org/10.1016/j.etap.2019.103209
APHA, AWWA, WEF (2012) Standard Methods for the Examination of Water and Wastewater, 22nd ed. APHA 800 I Street, NW, Washington, DC 20001–3710
Burress RM (1975) Enhancing bass production by the use of fish toxicants. In: Stroud RH, RH and Clepper H (eds) Black bass biology and management, Sport fishing Institute, Washington, DC pp 480–488. http://pubs.er.usgs.gov/publication/85606
Hamilton MA, Russo RC, Thurston RV (1977) Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ Sci Technol 11:714–719. https://doi.org/10.1021/es60130a004
Palermo FF, Risso WE, Simonato JD, Martinez CBR (2015) Bioaccumulation of nickel and its biochemical and genotoxic effects on juveniles of the neotropical fish Prochilodus lineatus. Ecotoxicol Environ Saf 116:19–28. https://doi.org/10.1016/j.ecoenv.2015.02.032
Satheeshkumar P, Ananthan G, Senthil Kumar D, Jagadeesan L (2012) Haematology and biochemical parameters of different feeding behaviour of teleost fishes from Vellar estuary, India. Comp Clin Pathol 21:1187–1191. https://doi.org/10.1007/s00580-011-1259-7
Schmid W (1975) The micronucleus test. Mutat Res Mutagen Relat Subj 31:9–15. https://doi.org/10.1016/0165-1161(75)90058-8
Fenech M, Jarvis LR, Morley AA (1988) Preliminary studies on scoring micronuclei by computerized image analysis. Mutat Res Mutagen Relat Subj 203:33–38. https://doi.org/10.1016/0165-1161(88)90005-2
Kakkar P, Das B, Viswanathan PN (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21(2):2–130. PMID: 6490072.
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Flohé L, Günzler AW (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–120. https://doi.org/10.1016/S0076-6879(84)05015-1
Carlberg I, Mannervik B (1975) Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 250:5475–5480. https://doi.org/10.1016/s0021-9258(19)41206-4
Ellman GL (1959) Tissue Su ~ yd ~ l Groups. Arch Biochem Biophys 82:70–77
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Awasthi Y, Ratn A, Prasad R et al (2018) An in vivo analysis of Cr 6+ induced biochemical, genotoxicological and transcriptional profiling of genes related to oxidative stress, DNA damage and apoptosis in liver of fish, Channa punctatus (Bloch, 1793). Aquat Toxicol 200:158–167. https://doi.org/10.1016/j.aquatox.2018.05.001
Kumar M, Gupta N, Ratn A, Awasthi Y, Prasad R, Trivedi A, Trivedi SP (2020) Biomonitoring of heavy metals in river ganga water, sediments, plant, and fishes of different trophic levels. Biol trace elem res 193(2):536–547. https://doi.org/10.1007/s12011-019-01736-0
Singh D, Nath K, Trivedi S, Sharma Y (2008) Impact of copper on haematological profile of freshwater fish, Channa punctatus. J Environ Biol 29:253–257
Liao J, Yang F, Chen H et al (2019) Effects of copper on oxidative stress and autophagy in hypothalamus of broilers. Ecotoxicol Environ Saf 185:109710. https://doi.org/10.1016/J.ECOENV.2019.109710
Zhong C-C, Zhao T, Hogstrand C et al (2021) Copper (Cu) induced changes of lipid metabolism through oxidative stress-mediated autophagy and Nrf2/PPARγ pathways. J Nutr Biochem:108883. https://doi.org/10.1016/J.JNUTBIO.2021.108883
Wang Y, Zhao H, Shao Y et al (2017) Copper or/and arsenic induce oxidative stress-cascaded, nuclear factor kappa B-dependent inflammation and immune imbalance, trigging heat shock response in the kidney of chicken. Oncotarget 8:98103–98116. https://doi.org/10.18632/ONCOTARGET.21463
Liu H, Guo H, Jian Z et al (2020) Copper induces oxidative stress and apoptosis in the mouse liver. Oxid Med Cell Longev 2020. https://doi.org/10.1155/2020/1359164
Trivedi PS, Ratn A, Yashika A et al (2021) In vivo assessment of dichlorvos induced histological and biochemical impairments coupled with expression of p53 responsive apoptotic genes in the liver and kidney of fish, Channa punctatus (Bloch, 1793). Comp Biochem Physiol C Toxicol Pharmacol 245:1–15. https://doi.org/10.1016/J.CBPC.2021.109032
Ratn A, Prasad R, Awasthi Y et al (2018) Zn 2+ induced molecular responses associated with oxidative stress, DNA damage and histopathological lesions in liver and kidney of the fish, Channa punctatus (Bloch, 1793). Ecotoxicol Environ Saf 151:10–20. https://doi.org/10.1016/j.ecoenv.2017.12.058
Sanchez W, Palluel O, Meunier L et al (2005) Copper-induced oxidative stress in three-spined stickleback: relationship with hepatic metal levels. Artic Environ Toxicol Pharmacol 19:177–183. https://doi.org/10.1016/j.etap.2004.07.003
Pandey S, Ahmad I, Parvez S et al (2001) (2001) Effect of endosulfan on antioxidants of freshwater fish Channa punctatus Bloch: 1. Protection against lipid peroxidation in liver by copper preexposure. Arch Environ Contam Toxicol 413(41):345–352. https://doi.org/10.1007/S002440010258
Atif F, Parvez S, Pandey S et al (2005) Modulatory effect of cadmium exposure on deltamethrin-induced oxidative stress in Channa punctata Bloch. Arch Environ Contam Toxicol 49:371–377. https://doi.org/10.1007/S00244-003-9231-4
Mandil R, Prakash A, Rahal A et al (2020) In vitro and in vivo effects of flubendiamide and copper on cyto-genotoxicity, oxidative stress and spleen histology of rats and its modulation by resveratrol, catechin, curcumin and α-tocopherol. Pharmacol Toxicol 29:1–17. https://doi.org/10.1186/s40360-020-00405-6
Yadav KK, Trivedi SP (2009) Sublethal exposure of heavy metals induces micronuclei in fish, Channa punctata. Chemosphere 77:1495–1500. https://doi.org/10.1016/j.chemosphere.2009.10.022
Yadav KK, Trivedi SP (2009) Chromosomal aberrations in a fish, Channa punctata after in vivo exposure to three heavy metals. Mutat Res Toxicol Environ Mutagen 678:7–12. https://doi.org/10.1016/j.mrgentox.2009.05.021
Kumar P, Kumar R, Nagpure NS et al (2012) Genotoxic and mutagenic assessment of hexavalent chromium in fish following in vivo chronic exposure. Hum Ecol Risk Assess 18:855–870. https://doi.org/10.1080/10807039.2012.688713
Mahboob S, Fares H, Al-Balwai A et al (2013) Investigation on the genotoxicity of mercuric chloride to freshwater clarias gariepinus. Pak Vet J 34:100–103
Dwivedi S, Tiwari V, Trivedi SP (2015) Arsenite induced genotoxic effect and its phytoremediation by Acacia catechu leaf extract in freshwater fish, Channa punctatus (Bloch). Int J Fish Aquat Stud 2:163–165
He C, Klionsky DJ (2009) Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:67. https://doi.org/10.1146/ANNUREV-GENET-102808-114910
Kim YC, Guan K-L (2015) mTOR: a pharmacologic target for autophagy regulation. J Clin Invest 125:25. https://doi.org/10.1172/JCI73939
Liao J, Yang F, Yu W et al (2020) Copper induces energy metabolic dysfunction and AMPK-mTOR pathway-mediated autophagy in kidney of broiler chickens. Ecotoxicol Environ Saf 206. https://doi.org/10.1016/J.ECOENV.2020.111366
Van Erp AC, Hoeksma D, Rebolledo RA et al (2017) The crosstalk between ROS and autophagy in the field of transplantation medicine. Oxid Med Cell Longev 2017. https://doi.org/10.1155/2017/7120962
Ye X, Zhou X-J, Zhang H (2018) Exploring the role of autophagy-related gene 5 (ATG5) yields important insights into autophagy in autoimmune/autoinflammatory diseases. Front Immunol 9:2334. https://doi.org/10.3389/FIMMU.2018.02334
Niture S, Lin M, Qi Q et al (2021) Role of autophagy in cadmium-induced hepatotoxicity and liver diseases. J Toxicol 2021. https://doi.org/10.1155/2021/9564297
Satyavarapu EM, Das R, Mandal C et al (2018) Autophagy-independent induction of LC3B through oxidative stress reveals its non-canonical role in anoikis of ovarian cancer cells. Cell Death Dis 910(9):1–18. https://doi.org/10.1038/s41419-018-0989-8
Sasai M, Sakaguchi N, Ma JS et al (2017) Essential role for GABARAP autophagy proteins in interferon-inducible GTPase-mediated host defense. Nat Immunol 18:899–910. https://doi.org/10.1038/NI.3767
Johansen T, Lamark T (2020) Selective autophagy: ATG8 family proteins, LIR motifs and cargo receptors. J Mol Biol 432:80–103. https://doi.org/10.1016/J.JMB.2019.07.016
He C, Bartholomew CR, Zhou W, Klionsky DJ (2009) Assaying autophagic activity in transgenic GFP-Lc3 and GFP- Gabarap zebra fish embryos. Autophagy 5(4):520–526. https://doi.org/10.4161/auto.5.4.7768
Weidberg H, Shvets E, Shpilka T et al (2010) LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J 29:1792–1802. https://doi.org/10.1038/EMBOJ.2010.74
Acknowledgements
We record our sincere thanks to the Department of Higher Education, Uttar Pradesh Government, U.P. India, for providing the Center of Excellence project 2019-20 vide grant no. 66/2019/1864/Satter-4-2019-4(24). We are also grateful to the Head, Department of Zoology, University of Lucknow, Lucknow (226007) for providing laboratory facilities.
Funding
This work was supported by the Department of Higher Education, Uttar Pradesh Government, U.P. India, vide grant no. 66/2019/1864/Satter-4–2019-4(24) and Dr. Manoj Kumar has received research support from the Department of Higher Education, Uttar Pradesh Government.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Experimental setup, data collection, and analysis were performed by Dr. Manoj Kumar, Ms. Shefalee Singh, Ms. Shikha Dwivedi, Dr. Abha Trivedi, Dr. Indrani Dubey, and Dr. Sunil P. Trivedi. The first draft of the manuscript was written by Dr. Manoj Kumar and Ms. Shefalee Singh and all authors commented on and revised the first draft of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Under the provisions of the committee for the purpose of control and supervision of experiments on animals (CPCSEA), the Government of India, an Institutional Animal Ethics Committee (IAEC) vide registration no. 1861/GO/Re/S/16/CPCSEA already exists in the University of Lucknow, Lucknow. We have followed the protocols mentioned therein CPCSEA for maintenance and experiment.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Cu2+ induces oxidative damage due to increased SOD, CAT, GSH, GR, and Gpx in liver and kidney tissues.
• Cu2+ downregulates expression of mTOR gene and promotes autophagy in liver and kidney tissues.
• A disturbed expression of various autophagy marker genes (LC3, ATG5, GABARAP, GATE16) after chronic exposure of Cu2+.
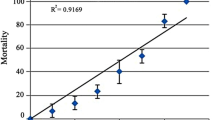
• Exposure of Channa punctatus to Cu2+ shows micronuclei formation in erythrocytes.
• A significant relation was observed between Cu2+-induced oxidative stress and autophagy.
Rights and permissions
About this article
Cite this article
Kumar, M., Singh, S., Dwivedi, S. et al. Copper-induced Genotoxicity, Oxidative Stress, and Alteration in Transcriptional Level of Autophagy-associated Genes in Snakehead Fish Channa punctatus. Biol Trace Elem Res 201, 2022–2035 (2023). https://doi.org/10.1007/s12011-022-03301-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03301-8