Abstract
COVID-19 is a severe acute respiratory syndrome caused by coronavirus 2 (SARS-CoV-2). Deficiency of zinc has been supposed to contribute to loss of smell and taste in COVID-19 patients. Our study aimed to assess the serum zinc levels among patients with COVID-19 of various severities, with and without olfaction dysfunction, and to evaluate the effect of zinc therapy in recovery of smell dysfunction among such patients. This study included 134 patients; real-time reverse transcription-polymerase chain reaction (rRT-PCR) proved SARS-CoV-2. Serum zinc levels were measured for all infected patients. One hundred and five patients were detected to have anosmia and/or hyposmia and were categorized randomly into 2 groups; the first group included 49 patients who received zinc therapy and the second group included 56 patients who did not received zinc. All patients were followed up for the recovery duration of olfactory and gustatory symptoms and duration of complete recovery of COVID-19. Olfactory dysfunction was reported in 105 patients (78.4%). Serum zinc levels were not significantly different between the patient subgroups regarding disease severity or the presence or absence of olfactory and/or gustatory dysfunction (p ˃ 0.05). The median duration of recovery of gustatory and/or olfactory function was significantly shorter among patients who received zinc therapy than those who did not received zinc (p < 0.001), while the median duration of complete recovery from COVID-19 was not significantly different among the two groups (p ˃ 0.05). Although the zinc status of COVID-19 patients did not exhibit a significant role in development of anosmia and/or hyposmia or disease severity, zinc therapy may have a significant role in shortening the duration of smell recovery in those patients without affecting the total recovery duration from COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 2019 novel coronavirus disease (COVID-19) is a viral infection, a severe acute respiratory syndrome caused by coronavirus 2 (SARS-CoV-2). The first case was diagnosed in Wuhan, China, in 2019 and caused multiorgan manifestation. Cases have been reported in more than 180 countries to the World Health Organization (WHO), including more than one million deaths [1]. The common symptoms of COVID-19 are fever, tiredness, dry cough, body pain, nasal congestion, rhinorrhea, pain in the throat or diarrhea, anosmia, and/or loss of taste [2].
Patients infected with SARS-CoV-2 presented with mild disease, and only 5% develop viral pneumonia and multiorgan failure [3].
Sinonasal conditions that impair the travel of odorants to the intact olfactory mucosa can result in anosmia [4]. Temporary anosmia can result from nasal congestion from various causes including respiratory viral infection [5]. In the pre-COVID-19 era, about 14 to 30% of all patients presented with olfactory impairment resulting from sinonasal diseases [6,7,8]. The incidence of olfactory dysfunction in COVID-19 is ranging from 68 to 85%, while taste dysfunction prevalence is ranging from 71 to 88.8% in SARS-CoV-2-infected patients [9].
Zinc is one of the most important trace metals in humans; it comes second to iron in concentration but with no special zinc store [10]. Plasma zinc concentration is about 1 μg/ml and represents about 0.1% of the whole body zinc [8, 11]. Internal homeostasis is regulated by 10 solute-linked carrier 30 (SLC30 or ZnT) exporters and 14 solute-linked carrier 39 (SLC39 or ZIP) importers [12, 13]. Zinc homeostasis is affected in overweight people; diabetic patients; ischemic heart diseases; drug intake as angiotensin-converting enzyme (ACE) inhibitors; angiotensin 2 receptor antagonists and thiazides; and intake of iron, calcium, and non-digestible plant ligands [14, 15]. Measuring of plasma zinc levels is a useful clinical test for zinc deficiency [16]. Zinc regulates the differentiation, proliferation, maturation, and function of lymphocytes and other leukocytes [13]. Also, zinc participates in viral recognition by the zinc finger protein ZCCHC3 which triggers the antiviral response [17, 18].
Zinc deficiency could be mild, moderate, or severe and present in about 17% of world population [19]. Mild and moderate zinc deficiencies are quit common globally [20]. Older people are more liable to zinc deficiency complications [19]. Skin diseases, growth retardation, high susceptibility to infections (including pneumonia), and others could be caused by severe zinc deficiency [21, 22]. In a clinical trial, daily intake of 20 mg zinc sulfate for 5 months reduced the morbidity of lower respiratory tract infection in comparison to placebo [23]. Interferon-alpha production is upregulated and its viral activity is increased with zinc intake. Also, SAR-CoV RNA polymerase activity was partially inhibited by zinc [24].
Chloroquine and hydroxychloroquine increase cellular uptake of zinc which may inhibit viral replication activity [25, 26]. Also, zinc is known to reduce angiotensin-converting enzyme 2 which is required for SARS-CoV-2 and SARS-CoV entry into target cells [27]. Deficiency of zinc has been supposed to contribute to loss of smell and taste in patients with COVID-19. Acute zinc deficiency occurs during acute infection which could lead to reduction in taste bud cell alkaline phosphatase activity, changing in salivary proteins containing zinc or leading to neurological dysfunction [28, 29]. The current research was designated to assess the relative frequency of olfactory disorders among patients with COVID-19 and to evaluate the serum levels of zinc and identify its possible relation with both the development of olfactory disorders and the disease severity, and to shed light on the possible role of zinc therapy regarding the improvement of impaired olfaction among patients with COVID-19.
Materials and Methods
Study Design and Setting
The current prospective clinical trial study included 134 patients with COVID-19, who were randomly selected from the Quarantine Department of Qena University Hospitals, Faculty of Medicine, South Valley University, Qena, Egypt, during the period from May 2020 to August 2020. Prior to start of the study, an institutional ethical committee approval was taken (ethical approval code: SVU-MED-MBC004-2020-04). A written informed consent was obtained from all participants in this study. Diagnosis of SARS-CoV-2 was based on history of epidemiologic exposure. Clinical manifestations include (1) respiratory symptoms and/or fever; (2) imaging features of SARS-CoV-2 infection; (3) total leucocytic counts showing normal or reduced lymphocyte count in early stage [30]; (4) imaging features of COVID-19, also diagnosed by real-time reverse transcription-polymerase chain reaction (rRT-PCR) in samples from respiratory tract swabs which were performed at Central Laboratories, Ministry of Health and Population, Cairo, Egypt.
Patients with history of nasal surgery, sinusitis, nasal polyposis allergic rhinitis, head injury, or chronic nasal disease were excluded. Also, patients with anosmia and/or hyposmia before the diagnosis of COVID-19 were excluded.
Data Collections
Demographic data were recorded for all patients including age, sex, BMI, comorbidities, and smoking. Full history was taken from all patients with special stress about the presence or absence of anosmia (loss of smell) or hyposmia (decrease sense of smell). The diagnosis of anosmia and hyposmia was according to the physician’s decision. In addition, proper examination of nasal cavity and paranasal sinuses was performed.
COVID-19 was categorized into mild, common, severe, and extremely severe in accordance with the 6th edition for Diagnostic Standards for COVID-19 [31]. Consequently, mild COVID-19 was considered to be associated with mild clinical symptoms, with no pneumonia manifestations on imaging. Patients with common COVID-19 had fever, respiratory tract, or other symptoms, and imaging that showed pneumonia. Severe COVID-19 was considered to meet one of the following conditions: (1) shortness of breath, 30 beats/min; (2) in the resting state, pulse oxygen saturation < 93%, arterial blood oxygen pressure (PaO2)/oxygen concentration (FiO2) < 300 mmHg; or (3) pulmonary imaging showed lesion progression > 50% within 24–48 h. Extremely severe COVID-19 needed to meet one of the following conditions: (1) development of respiratory failure requiring mechanical ventilation; (2) shock; (3) combined organ failure requiring ICU monitoring [32].
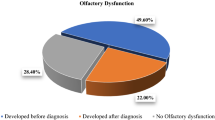
The patients with anosmia and/or hyposmia were divided randomly into two groups; the first group included 49 patients with olfactory dysfunction, who received zinc therapy (220 mg zinc sulfate equivocal to 50 mg elemental zinc twice daily [33]) plus the Egyptian protocol of treatment of COVID-19 and the second group included 56 patients with olfactory dysfunction, who received the Egyptian protocol of COVID-19 treatment without zinc therapy. Follow-up of all included patients until complete recovery of COVID-19 (pharyngeal swap becomes negative) and complete recovery of olfactory symptoms and the recovery durations for the included patients were recorded in days.
Biochemical and Molecular Assays
-
1.
Diagnostic kit (PCR-Fluorescence probing) of nucleic acid was used for the qualitative estimation of the ORF1ab and a specific conserved sequence of coding nucleocapsid protein N genes of novel coronavirus (2019-nCOV). This kit was supplied by Sansure Biotech Inc., Hunan Province, China, with catalog no. S3102E, using a 7500 real-time fluorescence quantitative RT-PCR system (Applied Biosystems, Foster City, CA, USA) to detect RNA through fluorescent signal changes [34].
-
2.
Serum levels of zinc were measured once for all patients prior to zinc therapy; 3 ml of peripheral venous blood samples was collected in plain collection tubes for serum recovery. Samples were left to clot for 30 min at 37 °C before centrifugation, and sera obtained were aliquoted into 1-ml cryotubes and stored at − 20 °C until biochemical assays of zinc. Zinc level was measured colorimetrically (Spectrum Diagnostics, Cairo, Egypt, Catalog No. 330001) [35,36,37,38,39].
Statistical Analysis
Analysis of data was performed using Statistical Program for Social Science (SPSS) version 24. The Kolmogorov-Smirnov test was used to check for data normality. Qualitative data was expressed as frequency, numbers, and percentages. Parametric quantitative data was expressed as mean ± standard deviation, while median and inter-quartile range used for non-parametric data. For comparison between the two groups, the chi-square test (χ2) was used for qualitative variables. Independent-samples t test was used to compare between two quantitative variables when normally distributed (parametric data), while for abnormally distributed variables (non-parametric data), the Mann-Whitney U test was used. One-way ANOVA test was used to compare more than two quantitative variables for parametric data. Probability (p value) > 0.05 was considered insignificant, p value < 0.05 was considered significant; and p value < 0.001 was considered as highly significant.
Results
Demographic Data of the Included COVID-19 Patients
The study included 134 patients diagnosed as COVID-19 who were categorized according to disease severity into mild (45 patients (24 males and 21 females)), common (57 patients (33 males and 24 females)), severe (21 patients (15 males and 7 females)), and extremely severe (11 patients with extremely severe symptoms (6 males and 5 females)). The mean ± SD of age (years) of patient groups were 31.8 ± 13.1, 47.8 ± 15.8, 59.1 ± 9.5, and 69.5 ± 6.5 respectively. The mean age was statistically significantly higher in patients with extremely severe disease than in others (p value < 0.001) (Table 1).
There were no significant differences regarding gender, BMI, and smoking status among COVID-19 patients of various diseases (p value > 0.05; Table 1). Both comorbidity frequency (diabetes mellitus, hypertension, and ischemic heart disease) and deaths were statistically significantly higher in severe and extremely severe COVID-19 patients as shown in Table 1 (p value < 0.05).
Smell Dysfunction Among Patients with COVID-19
In the current study, olfactory dysfunction (anosmia and hyposmia) was present in 105 out of the 134 patients (78.4%). Anosmia was reported in 80 patients (59.7%) and hyposmia in 25 patients (18.6%). There was no significant relation between olfactory dysfunction and severity of COVID-19 disease (Table 2).
Serum Zinc Levels Among the Included Patients with COVID-19
The mean serum zinc levels of all patients with different grades of severity are presented in Table 3. The serum zinc (mean ± SD, μg/ml) in patients with mild, common, severe, and extremely severe COVID-19 was 0.67 ± 0.18, 0.62 ± 0.14, 0.73 ± 0.18, and 0.72 ± 0.22. The difference between mean serum zinc levels among patients with COVID-19 of various severities was of marginal significance that may be explained by the small number of the included patients.
Zinc Levels and Olfactory Dysfunction Among Patients with COVID-19
Serum zinc levels in COVID-19 patients with anosmia and hyposmia are presented in Table 4. Despite lower serum zinc levels in patients with anosmia (0.59 ± 0.1 μg/dl) and in patients with hyposmia (0.57 ± 0.1 μg/dl) than patients without, this difference did not reach a significant level (p ˃ 0.05; Table 4). There was no significant difference between serum zinc levels of COVID-19 patients with anosmia (0.58 ± 0.1) vs. those with hyposmia (0.65 ± 0.1) (p ˃ 0.05).
Zinc Therapy and Recovery of COVID-19-Induced Olfactory Dysfunction
The median duration of recovery of olfactory function was 7 days (range 5–9 days) in COVID-19 patients who received zinc therapy which was significantly lower than in those who did not received (median 18 days with range 14–22 days) (p value < 0.05) (Table 5; Fig. 1). Additionally, zinc therapy did not influence the duration of complete recovery of COVID-19 disease among patients who received it (median 12 with range 8–17 days) vs. those who did not (median 12 with range 8–20 days) (p ˃ 0.05).
Discussion
COVID-19 (coronavirus disease) was first discovered in Wuhan, China, in December 2019 [40] with obscure characteristics of the disease. In Egypt, anosmia and hyposmia are common complaints in COVID-19 that interfere with quality of life. All age groups are at risk for infection and severe disease. However, the risk of fatal disease is highest in patients aged 65 years and older [41, 42]. The current study found that older age patients are more frequently affected by severe and extremely severe COVID-19, but young age patients are more frequently affected by mild to common disease. This comes in agreement with Liu et al. [43] who reported that elderly patients with COVID-19 are more likely to progress to severe COVID-19 disease in comparison with young- and middle-aged COVID-19 patients. Also, Yang et al. [44] and Mahase [45] reported similar findings, as in old aged, the cell-mediated immune function and humeral immune function reduced [46]. Other high-risk groups for COVID-19 are people with certain comorbidities as diabetes mellitus, hypertension, and ischemic heart diseases particularly when not controlled regardless of their age [47,48,49,50], which were in line with our findings which revealed that comorbidities (diabetes mellitus, hypertension, and ischemic heart disease) and deaths were significantly frequent in severe and extremely severe COVID-19 patients. Additionally, Marhl et al. [51] reported an increased risk of COVID-19 among diabetic patients because of the associated chronic inflammation, liver dysfunction, and dysregulation of angiotensin-converting enzyme 2 (ACE2). Also, Singh et al. [52] reported an increased severity and incidence of COVID-19 in diabetic patients. As regards smoking, there was no significant difference between different COVID-19 grades of severity which was in accordance with several researchers [53, 54].
In the current study, the frequency of anosmia and hyposmia among the included COVID-19 patients was 59.7% and 18.6% respectively. Online cross-sectional survey by Yan et al. noticed that 40 patients out of 59 patients tested positive for COVID-19 (68%) reported loss of smell, which was near to the results of the present study.
Zinc has been reported to inhibit coronavirus RNA polymerase activity in vitro [24], and is claimed to play a role in antiviral immune responses. SARS-CoV-2 infection depends on the metabolism of the host cell. Zinc is claimed to have antiviral effects demonstrated in various cases [54,55,56]. Examples include Coronaviridae, papilloma virus, picornavirus, metapneumovirus, herpes simplex virus, rhinovirus, varicella-zoster virus, human immunodeficiency virus (HIV), respiratory syncytial virus, and hepatitis C virus [57, 58]. Viral fusion with the host cell membrane could be prevented by zinc; also, it impairs the function of viral polymerase.
Although the current study revealed no significant difference in serum zinc levels between patients with different COVID-19 grades of severity, p value was marginal (p = 0.084). To obtain conclusions, a larger study is necessary. It could be just by the inadequate statistical power due to the small sample size. A study reported the important role of zinc in reducing duration of symptoms of common cold, but not its severity [28].
Our results revealed significantly lower serum zinc levels in patients with olfactory impairment than those without, but did not reach a significant level. Pisano and Hilas reported that zinc deficiency is linked to taste and smell disorders in COVID-19 patients [59].
The findings of the current study showed that COVID-19 patients who received zinc therapy exhibited significantly lower duration of smell recovery than those who did not, without significant difference regarding the total recovery duration of COVID-19. This indicates that zinc therapy could improve the associated smell abnormality, but not affect the COVID-19 disease outcome. In a placebo-controlled randomized trial investigating the effect of zinc supplementation in treating smell dysfunction post-chemotherapy, Lyckholm and his colleges reported no significant value of zinc therapy in improving smell dysfunction [60]. In COVID-19 patients, deficient formation of type I and type II interferons was reported [59]. Zinc supplementation is claimed to reconstitute secretion of human interferon-alpha (IFN-a); it is suspected to have antiviral actions as in rhinovirus-infected cells [61, 62].
Conclusions
Zinc status could not have a role in development of anosmia and/or hyposmia among COVID-19 patients. Zinc therapy significantly reduced the recovery duration of anosmia/and or hyposmia in those patients without affecting the total recovery duration of COVID-19. Further larger scale studies should be performed to evaluate the possible role of zinc on the immune system in SARS-CoV-2 infection.
References
WHO Coronavirus Disease (COVID-19) Dashboard. Available https://covid19.who.int/. Accessed October 11, 2020
Eurosurveillance ET (2020) Updated rapid risk assessment from ECDC on coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK. Euro Surveill 25:2003121. https://doi.org/10.2807/1560-7917.ES.2020.25.10.2003121
Wu Z, McGoogan JM (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. https://doi.org/10.1001/jama.2020.26
Cain WS, Gent JF, Goodspeed RB, Leonard G (1988) Evaluation of olfactory dysfunction in the Connecticut Chemosensory Clinical Research Center. Laryngoscope 98(1):83–88
Miwa T, Furukawa M, Tsukatani T, Costanzo RM, DiNardo LJ, Reiter ER (2001) Impact of olfactory impairment on quality of life and disability. Arch Otolaryngol Head Neck Surg 127(5):497–503
Seiden AM, Duncan HJ (2001) The diagnosis of a conductive olfactory loss. Laryngoscope 111(1):9–14
Temmel AF, Quint C, Schickinger-Fischer B, Klimek L, Stoller E, Hummel T (2002) Characteristics of olfactory disorders in relation to major causes of olfactory loss. Arch Otolaryngol Head Neck Surg 128(6):635–641
Wessels I, Maywald M, Rink L (2017) Zinc as a gatekeeper of immune function. Nutrients 9:1286. https://doi.org/10.3390/nu9121286
Lozada-Nur F, Chainani-Wu N, Fortuna G, Sroussi H (2020) Dysgeusia in COVID-19: possible mechanisms and implications. Oral Surg Oral Med Oral Pathol Oral Radiol 130(3):344–346
Gammoh NZ, Rink L (2017) Zinc in infection and inflammation. Nutrients 9:624. https://doi.org/10.3390/nu9060624
Maywald M, Wessels I, Rink L (2017) Zinc signals and immunity. Int J Mol Sci 18:2222. https://doi.org/10.3390/ijms18102222
Huang L, Tepaamorndech S (2013) The SLC30 family of zinc transporters – a review of current understanding of their biological and pathophysiological roles. Mol Asp Med 34:548–560. https://doi.org/10.1016/j.mam.2012.05.008
Jeong J, Eide DJ (2013) The SLC39 family of zinc transporters. Mol Asp Med 34:612–619. https://doi.org/10.1016/j.mam.2012.05.011
Olechnowicz J, Tinkov A, Skalny A, Suliburska J (2018) Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J Physiol Sci 68:19–31. https://doi.org/10.1007/s12576-017-0571-7
Braun LA, Rosenfeldt F (2013) Pharmaco-nutrient interactions - a systematic review of zinc and antihypertensive therapy. Int J Clin Pract 67:717–725. https://doi.org/10.1111/ijcp.12040
Lowe NM, Fekete K, Decsi T (2009) Methods of assessment of zinc status in humans: a systematic review. Am J Clin Dermatol 89:2040S–2051S. https://doi.org/10.3945/ajcn.2009.27230G
Lian H, Zang R, Wei J, Ye W, Hu MM, Chen YD, Zhang XN, Guo Y, Lei CQ, Yang Q, Luo WW, Li S, Shu HB (2018) The zinc-finger protein ZCCHC3 binds RNA and facilitates viral RNA sensing and activation of the RIG-I-like receptors. Immunity 49(3):438–448.e5. https://doi.org/10.1016/j.immuni.2018.08.014
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–701. https://doi.org/10.1016/j.cell.2006.02.015
Yasuda H, Tsutsui T (2016) Infants and elderlies are susceptible to zinc deficiency. Sci Rep 6:21850. https://doi.org/10.1038/srep21850
Sandstead HH (1991) Zinc deficiency. A public health problem? Am J Dis Child 145:853–859. https://doi.org/10.1001/archpedi.1991.02160080029016
Prasad AS (2013) Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr 4:176–190. https://doi.org/10.3945/an.112.003210
Haase H, Rink L (2014) Multiple impacts of zinc on immune function. Metallomics. 6:1175–1180. https://doi.org/10.1039/c3mt00353a
Malik A, Taneja DK, Devasenapathy N, Rajeshwari K (2014) Zinc supplementation for prevention of acute respiratory infections in infants: a randomized controlled trial. Indian Pediatr 51:780–784. https://doi.org/10.1007/s13312-014-0503-z
te Velthuis AJ, van denWorm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ (2010) Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog e1001176:6. https://doi.org/10.1371/journal.ppat.1001176
Xue J, Moyer A, Peng B, Wu J, Hannafon BN, Ding WQ (2014) Chloroquine is a zinc ionophore. PLoS One 9:e109180. https://doi.org/10.1371/journal.pone.0109180
Devaux CA, Rolain JM, Colson P, Raoult D (2020) New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents 55:105938. https://doi.org/10.1016/j.ijantimicag.2020.105938
Speth R, Carrera E, Jean-Baptiste JA, Linares A (2014) Concentration dependent effects of zinc on angiotensin-converting enzyme-2 activity (1067.4). FASEB J 28(1):1067. https://doi.org/10.1096/fasebj.28.1_supplement.1067.4
Pisano M, Hilas O (2016) Zinc and taste disturbances in older adults: a review of the literature. Consult Pharm 31:267–270. https://doi.org/10.4140/TCP.n.2016.267
Vaira LA, Salzano G, Deiana G, De Riu G (2020) Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope 130:1787. https://doi.org/10.1002/lary.28692
Wang YY, Jin YH, Ren XQ, Li YR, Zhang XC, Zeng XT, Wang XH, Zhongnan Hospital of Wuhan University Novel Coronavirus Management and Research Team (2020) Updating the diagnostic criteria of COVID-19 “suspected case” and “confirmed case” is necessary. Mil Med Res 7(1):17. https://doi.org/10.1186/s40779-020-00245-9
Medicine. GOoNHCOoSAoTC. Notice on the issuance of a program for the diagnosis and treatment of novel coronavirus (2019-nCoV) infected pneumonia (trial sixth edition). (2020-02-19) [EB/OL] http://yzssatcmgovcn/zhengcewenjian/2020-02-19/13221html
Li L, Li R, Wu Z, Yang X, Zhao M, Liu J, Chen D (2020) Therapeutic strategies for critically ill patients with COVID-19. Ann Intensive Care 10(1):45. https://doi.org/10.1186/s13613-020-00661-z
COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/. Accessed [17 July, 2020]
Ghweil AA, Hassan MH, Khodeary A, Mohamed AO, Mohammed HM, Abdelazez AA, Osman HA, Bazeed SES (2020) Characteristics, outcomes and indicators of severity for COVID-19 among sample of ESNA Quarantine Hospital’s Patients, Egypt: a retrospective study. Infect Drug Resist 13:2375–2383
El-Masry HMA, Sadek AA, Hassan MH, Ameen HH, Ahmed HA (2018) Metabolic profile of oxidative stress and trace elements in febrile seizures among children. Metab Brain Dis 33:1509–1515
Sakhr HM, Hassan MH, Desoky T (2020) Possible associations of disturbed neurometals and ammonia with glycaemic control in type 1 diabetic children with attention deficit hyperactivity disorder. Biol Trace Elem Res 198:68–76
Ahmed AE, Hassan MH, Rashwan NI, Sayed MM, Meki AMA (2018) Myocardial injury induced by scorpion sting envenoming and evidence of oxidative stress in Egyptian children. Toxicon 153:72–77
Ahmed AE, Abd-Elmawgood EA, Hassan MH (2017) Circulating protein carbonyls, antioxidant enzymes and related trace minerals among preterms with respiratory distress syndrome. J Clin Diagn Res 11:17–21
Saleem TH, Okasha M, Ibrahim HM, Abu El-Hamd M, Fayed HM, Hassan MH (2020) Biochemical assessments of seminal plasma zinc, testis-expressed sequence 101 and free amino acids and their correlations with reproductive hormones in male infertility. Biol Trace Elem Res. https://doi.org/10.1007/s12011-020-02310-9
Zhang J, Wang X, Jia X, Li J, Hu K, Chen G, Wei J, Gong Z, Zhou C, Yu H, Yu M, Lei H, Cheng F, Zhang B, Xu Y, Wang G, Dong W (2020) Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect 26(6):767–772. https://doi.org/10.1016/j.cmi.2020.04.012.
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 180(7):934–943. https://doi.org/10.1001/jamainternmed.2020.0994.
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, DSC H, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for Covid-19 (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382(18):1708–1720. https://doi.org/10.1056/NEJMoa2002032
Liu K, Chen Y, Lin R, Han K (2020) Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Inf Secur. https://doi.org/10.1016/j.jinf.2020.03.005
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y (2020) Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 94:91–95. https://doi.org/10.1016/j.ijid.2020.03.017.
Mahase E (2020) Covid-19: death rate is 0.66% and increases with age, study estimates. BMJ 369:m1327. https://doi.org/10.1136/bmj.m1327
Zhou F, Yu T, Du R, Fan G, Liu Y (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229):1054e62. https://doi.org/10.1016/S0140-6736(20)30566-3
Cai Q, Chen F, Wang T, Luo F, Liu X, Wu Q, He Q, Wang Z, Liu Y, Liu L, Chen J, Xu L (2020) Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care 43(7):1392–1398. https://doi.org/10.2337/dc20-0576
Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): people who are at higher risk for severe illness. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html. Accessed April 8, 2020
Garg S, Kim L, Whitaker M, O’Halloran A, Cummings C, Holstein R, Prill M, Chai SJ, Kirley PD, Alden NB, Kawasaki B, Yousey-Hindes K, Niccolai L, Anderson EJ, Openo KP, Weigel A, Monroe ML, Ryan P, Henderson J, Kim S, Como-Sabetti K, Lynfield R, Sosin D, Torres S, Muse A, Bennett NM, Billing L, Sutton M, West N, Schaffner W, Talbot HK, Aquino C, George A, Budd A, Brammer L, Langley G, Hall AJ, Fry A (2020) Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep 69(15):458–464. https://doi.org/10.15585/mmwr.mm6915e3
Aly MH, Rahman SS, Ahmed WA, Alghamedi MH, Al Shehri AA, Alkalkami AM, Hassan MH (2020) Indicators of critical illness and predictors of mortality in COVID-19 patients. Infect Drug Resist 13:1995–2000. https://doi.org/10.2147/IDR.S261159.
Marhl M, Grubelnik V, Magdič M, Markovič R (2020) Diabetes and metabolic syndrome as risk factors for COVID-19. Diabetes Metab Syndr 14(4):671–677. https://doi.org/10.1016/j.dsx.2020.05.013
Singh AK, Gupta R, Ghosh A, Misra A (2020) Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr 14(4):303–310. https://doi.org/10.1016/j.dsx.2020.04.004
Cai G, Cui X, Zhu X, Zhou J (2020) A hint on the COVID-19 risk: population disparities in gene expression of three receptors of SARS-CoV. Preprints, 2020020408. https://doi.org/10.20944/preprints202002.0408.v1
Vardavas CI, Nikitara K (2020) COVID-19 and smoking: a systematic review of the evidence. Tob Induc Dis 18:20. https://doi.org/10.18332/tid/119324
Ishida T (2019) Review on the role of Zn2+ ions in viral pathogenesis and the effect of Zn2+ Ions for host cell-virus growth inhibition. AJBSR 2:28–37. https://doi.org/10.34297/AJBSR.2019.02.000566
Skalny AV, Rink L, Ajsuvakova OP, Aschner M, Gritsenko VA, Alekseenko SI, Svistunov AA, Petrakis D, Spandidos DA, Aaseth J, Tsatsakis A, Tinkov AA (2020) Zinc and respiratory tract infections: perspectives for COVID-19 (review). Int J Mol Med 46(1):17–26. https://doi.org/10.3892/ijmm.2020.4575.
Cai H, Zhang Y, Ma Y, Sun J, Liang X, Li J (2015) Zinc binding activity of human metapneumovirus M2-1 protein is indispensable for viral replication and pathogenesis in vivo. J Virol 89:6391–6405. https://doi.org/10.1128/JVI.03488-14
Kumar A, Kubota Y, Chernov M, Kasuya H (2020) Potential role of zinc supplementation in prophylaxis and treatment of COVID-19. Med Hypotheses 144:109848. https://doi.org/10.1016/j.mehy.2020.109848.
Lyckholm L, Heddinger SP, Parker G, Coyne PJ, Ramakrishnan V, Smith TJ, Henkin RI (2012) A randomized, placebo controlled trial of oral zinc for chemotherapy-related taste and smell disorders. J Pain Palliat Care Pharmacother 26(2):111–114. https://doi.org/10.3109/15360288.2012.676618.
Berg K, Bolt G, Andersen H, Owen TC (2001) Zinc potentiates the antiviral action of human IFN-alpha tenfold. J Interf Cytokine Res 21:471–474. https://doi.org/10.1089/10799900152434330
Cakman I, Kirchner H, Rink L (1997) Zinc supplementation reconstitutes the production of interferon-alpha by leukocytes from elderly persons. J Interf Cytokine Res 17:469–472. https://doi.org/10.1089/jir.1997.17.469
Mayor-Ibarguren A, Busca-Arenzana C, Robles-Marhuenda Á (2020) A hypothesis for the possible role of zinc in the immunological pathways related to COVID-19 infection. Front Immunol 11:1736. https://doi.org/10.3389/fimmu.2020.01736
Funding
The current research was funded by the authors themselves.
Author information
Authors and Affiliations
Contributions
Study concept and design: AAA, MHH, AAG, ZFA, and SESB; patient selection and clinical evaluation and follow-up of patients: AAA, ZFA, AR, and MKE; blood sampling, molecular and biochemical assays: MHH and AK; statistical analysis: AAA, MHH, SESB, AR, MKE, and MAAS; literature research: AAA, SESB, MHH, MAAS, AR, and ZFA; first manuscript drafting: AAA, SESB, and MHH. The authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no conflict of interest.
Ethics Approval and Consent to Participate
The study was approved by the local Ethics Committee of Medical Research of the Faculty of Medicine, South Valley University, Qena, Egypt (ethical approval code: SVU-MED-MBC004-2020-04), and was conducted in accordance with the Declaration of Helsinki. Informed written consent was obtained from every participant who was anonymously enrolled.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdelmaksoud, A.A., Ghweil, A.A., Hassan, M.H. et al. Olfactory Disturbances as Presenting Manifestation Among Egyptian Patients with COVID-19: Possible Role of Zinc. Biol Trace Elem Res 199, 4101–4108 (2021). https://doi.org/10.1007/s12011-020-02546-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02546-5