Abstract
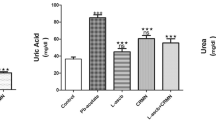
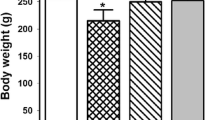
In this study, the protective effects of chrysin (CR) on lead acetate (PbAc)-induced renal toxicity in Sprague-Dawley rats were investigated with biochemical, histopathological, and immunohistochemical methods. In the study, rats were given orally at 30 mg/kg/body weight (BW) PbAc after CR of 25 and 50 mg/kg/BW was administered to them orally (a total of 7 administrations for 7 days). The results showed that CR reduced urea and creatinine levels by alleviating PbAc-induced kidney damage. It was determined that CR decreases PbAc-induced lipid peroxidation due to its antioxidant properties and increases catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx) activities, and glutathione (GSH) levels. It was also detected that CR protects DNA from the toxic effects of PbAc and reduces 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels. Biochemical and immunohistochemical findings demonstrated that CR had anti-inflammatory and antiapoptotic effects and reduced nuclear factor kappa-B (NF-κB), interleukin-33 (IL-33), prostaglandin-E2 (PGE-2), tumor necrosis factor-α (TNF-α), p53 levels, and the activities of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), which were increased with PbAc administration. Moreover, CR was found to increase the levels of aquaporin-1 (AQP-1) and nephrine in PbAc-induced kidney tissue. CR decreased the contents of lead (Pb), zinc (Zn), iron (Fe), sodium (Na), and copper (Cu) and increased those of potassium (K) calcium (Ca) in renal tissue. These results indicated that CR considerably alleviates kidney toxicity caused by PbAc.







Similar content being viewed by others
References
Phyu MP, Tangpong J (2014) Neuroprotective effects of xanthone derivative of Garcinia mangostana against lead-induced acetylcholinesterase dysfunction and cognitive impairment. Food Chem Toxicol 70:151–156. https://doi.org/10.1016/j.fct.2014.04.035
Khalil SR, Khalifa HA, Abdel-Motal SM, Mohammed HH, Elewa YHA, Mahmoud HA (2018) Spirulina platensis attenuates the associated neurobehavioral and inflammatory response impairments in rats exposed to lead acetate. Ecotoxicol Environ Saf 157:255–265. https://doi.org/10.1016/j.ecoenv.2018.03.068
Soleimani E, Goudarzi I, Abrari K, Lashkarbolouki T (2016) The combined effects of developmental lead and ethanol exposure on hippocampus dependent spatial learning and memory in rats: role of oxidative stress. Food Chem Toxicol 96:263–272. https://doi.org/10.1016/j.fct.2016.07.009
Khalil SR, Elhady WM, Elewa YHA, Abd El-Hameed NE, Ali SA (2018) Possible role of Arthrospira platensis in reversing oxidative stress-mediated liver damage in rats exposed to lead. Biomed Pharmacother 97:1259–1268. https://doi.org/10.1016/j.biopha.2017.11.045
Shojaeepour S, Fazeli M, Oghabian Z, Pourgholi L, Mandegary A (2018) Oxidative stress in opium users after using lead-adulterated opium: the role of genetic polymorphism. Food Chem Toxicol 120:571–577. https://doi.org/10.1016/j.fct.2018.07.061
Song XB, Liu G, Liu F, Yan ZG, Wang ZY, Liu ZP, Wang L (2017) Autophagy blockade and lysosomal membrane permeabilization contribute to lead-induced nephrotoxicity in primary rat proximal tubular cells. Cell Death Dis 8(6):e2863. https://doi.org/10.1038/cddis.2017.262
Abd El-Hack ME, Abdelnour SA, Abd El-Moneim AEE, Arif M, Khafaga A, Shaheen H, Samak D, Swelum AA (2019) Putative impacts of phytogenic additives to ameliorate lead toxicity in animal feed. Environ Sci Pollut Res Int 26(23):23209–23218. https://doi.org/10.1007/s11356-019-05805-8
Oyagbemi AA, Omobowale TO, Akinrinde AS, Saba AB, Ogunpolu BS, Daramola O (2015) Lack of reversal of oxidative damage in renal tissues of lead acetate-treated rats. Environ Toxicol 30(11):1235–1243. https://doi.org/10.1002/tox.21994
Patrick L (2006) Lead toxicity, a review of the literature. Part 1: exposure, evaluation, and treatment. Altern Med Rev 11(1):2–22
Matovic V, Buha A, Ethukic-Cosic D, Bulat Z (2015) Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem Toxicol 78:130–140. https://doi.org/10.1016/j.fct.2015.02.011
Farmand F, Ehdaie A, Roberts CK, Sindhu RK (2005) Lead-induced dysregulation of superoxide dismutases, catalase, glutathione peroxidase, and guanylate cyclase. Environ Res 98(1):33–39. https://doi.org/10.1016/j.envres.2004.05.016
Liu J, Jia DY, Cai SZ, Li CP, Zhang MS, Zhang YY, Yan CH, Wang YP (2015) Mitochondria defects are involved in lead-acetate-induced adult hematopoietic stem cell decline. Toxicol Lett 235(1):37–44. https://doi.org/10.1016/j.toxlet.2015.03.007
Dobrakowski M, Pawlas N, Kasperczyk A, Kozlowska A, Olewinska E, Machon-Grecka A, Kasperczyk S (2017) Oxidative DNA damage and oxidative stress in lead-exposed workers. Hum Exp Toxicol 36(7):744–754. https://doi.org/10.1177/0960327116665674
Caglayan C, Kandemir FM, Darendelioglu E, Yildirim S, Kucukler S, Dortbudak MB (2019) Rutin ameliorates mercuric chloride-induced hepatotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis. J Trace Elem Med Biol 56:60–68. https://doi.org/10.1016/j.jtemb.2019.07.011
Alcaraz-Contreras Y, Mendoza-Lozano RP, Martinez-Alcaraz ER, Martinez-Alfaro M, Gallegos-Corona MA, Ramirez-Morales MA, Vazquez-Guevara MA (2016) Silymarin and dimercaptosuccinic acid ameliorate lead-induced nephrotoxicity and genotoxicity in rats. Hum Exp Toxicol 35(4):398–403. https://doi.org/10.1177/0960327115591373
Andersen O, Aaseth J (2016) A review of pitfalls and progress in chelation treatment of metal poisonings. J Trace Elem Med Biol 38:74–80. https://doi.org/10.1016/j.jtemb.2016.03.013
BaSalamah MA, Abdelghany AH, El-Boshy M, Ahmad J, Idris S, Refaat B (2018) Vitamin D alleviates lead induced renal and testicular injuries by immunomodulatory and antioxidant mechanisms in rats. Sci Rep 8(1):4853. https://doi.org/10.1038/s41598-018-23258-w
Benzer F, Kandemir FM, Kucukler S, Comakli S, Caglayan C (2018) Chemoprotective effects of curcumin on doxorubicin-induced nephrotoxicity in wistar rats: by modulating inflammatory cytokines, apoptosis, oxidative stress and oxidative DNA damage. Arch Physiol Biochem 124(5):448–457. https://doi.org/10.1080/13813455.2017.1422766
Caglayan C, Kandemir FM, Yildirim S, Kucukler S, Eser G (2019) Rutin protects mercuric chloride-induced nephrotoxicity via targeting of aquaporin 1 level, oxidative stress, apoptosis and inflammation in rats. J Trace Elem Med Biol 54:69–78. https://doi.org/10.1016/j.jtemb.2019.04.007
Kuzu M, Kandemir FM, Yildirim S, Kucukler S, Caglayan C, Turk E (2018) Morin attenuates doxorubicin-induced heart and brain damage by reducing oxidative stress, inflammation and apoptosis. Biomed Pharmacother 106:443–453. https://doi.org/10.1016/j.biopha.2018.06.161
Kandemir FM, Kucukler S, Caglayan C, Gur C, Batil AA, Gülçin İ (2017) Therapeutic effects of silymarin and naringin on methotrexate-induced nephrotoxicity in rats: biochemical evaluation of anti-inflammatory, antiapoptotic, and antiautophagic properties. J Food Biochem 41(5):e12398
Benzer F, Kandemir FM, Ozkaraca M, Kucukler S, Caglayan C (2018) Curcumin ameliorates doxorubicin-induced cardiotoxicity by abrogation of inflammation, apoptosis, oxidative DNA damage, and protein oxidation in rats. J Biochem Mol Toxicol 32(2). https://doi.org/10.1002/jbt.22030
Kandemir FM, Yildirim S, Kucukler S, Caglayan C, Mahamadu A, Dortbudak MB (2018) Therapeutic efficacy of zingerone against vancomycin-induced oxidative stress, inflammation, apoptosis and aquaporin 1 permeability in rat kidney. Biomed Pharmacother 105:981–991. https://doi.org/10.1016/j.biopha.2018.06.048
Kandemir FM, Ozkaraca M, Küçükler S, Caglayan C, Hanedan B (2018) Preventive effects of hesperidin on diabetic nephropathy induced by streptozotocin via modulating TGF-β1 and oxidative DNA damage. Toxin Rev 37(4):287–293
Celik H, Kucukler S, Comakli S, Ozdemir S, Caglayan C, Yardim A, Kandemir FM (2019) Morin attenuates ifosfamide-induced neurotoxicity in rats via suppression of oxidative stress, neuroinflammation and neuronal apoptosis. Neurotoxicology 76:126–137. https://doi.org/10.1016/j.neuro.2019.11.004
Hanedan B, Ozkaraca M, Kirbas A, Kandemir FM, Aktas MS, Kilic K, Comakli S, Kucukler S, Bilgili A (2018) Investigation of the effects of hesperidin and chrysin on renal injury induced by colistin in rats. Biomed Pharmacother 108:1607–1616. https://doi.org/10.1016/j.biopha.2018.10.001
Samarghandian S, Farkhondeh T, Azimi-Nezhad M (2017) Protective effects of chrysin against drugs and toxic agents. Dose-Response 15(2):1559325817711782. https://doi.org/10.1177/1559325817711782
Aksu EH, Ozkaraca M, Kandemir FM, Omur AD, Eldutar E, Kucukler S, Comakli S (2016) Mitigation of paracetamol-induced reproductive damage by chrysin in male rats via reducing oxidative stress. Andrologia 48(10):1145–1154. https://doi.org/10.1111/and.12553
Tahir M, Sultana S (2011) Chrysin modulates ethanol metabolism in Wistar rats: a promising role against organ toxicities. Alcohol Alcohol 46(4):383–392. https://doi.org/10.1093/alcalc/agr038
Eldutar E, Kandemir FM, Kucukler S, Caglayan C (2017) Restorative effects of chrysin pretreatment on oxidant-antioxidant status, inflammatory cytokine production, and apoptotic and autophagic markers in acute paracetamol-induced hepatotoxicity in rats: an experimental and biochemical study. J Biochem Mol Toxicol 31(11). https://doi.org/10.1002/jbt.21960
Mantawy EM, El-Bakly WM, Esmat A, Badr AM, El-Demerdash E (2014) Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur J Pharmacol 728:107–118. https://doi.org/10.1016/j.ejphar.2014.01.065
Aksu EH, Kandemir FM, Kucukler S, Mahamadu A (2018) Improvement in colistin-induced reproductive damage, apoptosis, and autophagy in testes via reducing oxidative stress by chrysin. J Biochem Mol Toxicol 32(11):e22201. https://doi.org/10.1002/jbt.22201
Zheng X, Meng WD, Xu YY, Cao JG, Qing FL (2003) Synthesis and anticancer effect of chrysin derivatives. Bioorg Med Chem Lett 13(5):881–884. https://doi.org/10.1016/s0960-894x(02)01081-8
Ha SK, Moon E, Kim SY (2010) Chrysin suppresses LPS-stimulated proinflammatory responses by blocking NF-kappaB and JNK activations in microglia cells. Neurosci Lett 485(3):143–147. https://doi.org/10.1016/j.neulet.2010.08.064
Mani R, Natesan V (2018) Chrysin: sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 145:187–196. https://doi.org/10.1016/j.phytochem.2017.09.016
Tsuji PA, Walle T (2008) Cytotoxic effects of the dietary flavones chrysin and apigenin in a normal trout liver cell line. Chem Biol Interact 171(1):37–44. https://doi.org/10.1016/j.cbi.2007.08.007
Asad A, Hamid S, Qama K (2018) Effect of Lead acetate on basement membrane of seminiferous tubules of adult rat testis and protective effects of Ficus carica: a histological study. J Coll Physicians Surg Pak 28(10):731–734 3010
Temel Y, Kucukler S, Yildirim S, Caglayan C, Kandemir FM (2020) Protective effect of chrysin on cyclophosphamide-induced hepatotoxicity and nephrotoxicity via the inhibition of oxidative stress, inflammation, and apoptosis. Naunyn Schmiedeberg's Arch Pharmacol 393(3):325–337. https://doi.org/10.1007/s00210-019-01741-z
Talke H, Schubert GE (1965) Enzymatic urea determination in the blood and serum in the Warburg optical test. Klin Wochenschr 43:174–175. https://doi.org/10.1007/BF01484513
Newman D (1999) Renal function and nitrogen metabolites. Tietz textbook of clinical chemistry:1204-1270
Placer ZA, Cushman LL, Johnson BC (1966) Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem 16(2):359–364. https://doi.org/10.1016/0003-2697(66)90167-9
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34(3):497–500
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Lawrence RA, Burk RF (1976) Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun 71(4):952–958. https://doi.org/10.1016/0006-291x(76)90747-6
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25(1):192–205. https://doi.org/10.1016/0003-2697(68)90092-4
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193(1):265–275
Moustafa GG, Khalil S, Hussein MM, Labib M (2012) The cytotoxic and ultrastrctural perturbations of aluminum exposed nile catfish with special reference to the mitigating effect of vitamin C
Khalil SR, Hussein MM (2015) Neurotransmitters and neuronal apoptotic cell death of chronically aluminum intoxicated Nile catfish (Clarias gariepinus) in response to ascorbic acid supplementation. Neurotoxicology 51:184–191. https://doi.org/10.1016/j.neuro.2015.09.008
Abdel-Moneim AM, El-Toweissy MY, Ali AM, Allah AAMA, Darwish HS, Sadek IA (2015) Curcumin ameliorates lead (Pb2+)-induced hemato-biochemical alterations and renal oxidative damage in a rat model. Biol Trace Elem Res 168(1):206–220
Qi SS, Zheng HX, Jiang H, Yuan LP, Dong LC (2020) Protective effects of chromium picolinate against diabetic-induced renal dysfunction and renal fibrosis in streptozotocin-induced diabetic rats. Biomolecules 10(3). https://doi.org/10.3390/biom10030398
Soussi A, Gargouri M, Akrouti A, El Feki A (2018) Antioxidant and nephro-protective effect of Juglans regia vegetable oil against lead-induced nephrotoxicity in rats and its characterization by GC-MS. EXCLI J 17:492–504. https://doi.org/10.17179/excli2018-1235
Köksal E, Bursal E, Gülçin İ, Korkmaz M, Çağlayan C, Gören AC, Alwasel SH (2017) Antioxidant activity and polyphenol content of Turkish thyme (Thymus vulgaris) monitored by liquid chromatography and tandem mass spectrometry. Int J Food Prop 20(3):514–525
Taslimi P, Kandemir FM, Demir Y, Ileriturk M, Temel Y, Caglayan C, Gulcin I (2019) The antidiabetic and anticholinergic effects of chrysin on cyclophosphamide-induced multiple organ toxicity in rats: pharmacological evaluation of some metabolic enzyme activities. J Biochem Mol Toxicol:e22313. doi:https://doi.org/10.1002/jbt.22313
Zheng HX, Qi SS, He J, Hu CY, Han H, Jiang H, Li XS (2020) Cyanidin-3-glucoside from black rice ameliorates diabetic nephropathy via reducing blood glucose, suppressing oxidative stress and inflammation, and regulating transforming growth factor beta1/Smad expression. J Agric Food Chem 68(15):4399–4410. https://doi.org/10.1021/acs.jafc.0c00680
Gao J, Wang X, Chang Y, Zhang J, Song Q, Yu H, Li X (2006) Acetazolamide inhibits osmotic water permeability by interaction with aquaporin-1. Anal Biochem 350(2):165–170. https://doi.org/10.1016/j.ab.2006.01.003
Kishore BK, Krane CM, Di Iulio D, Menon AG, Cacini W (2000) Expression of renal aquaporins 1, 2, and 3 in a rat model of cisplatin-induced polyuria. Kidney Int 58(2):701–711. https://doi.org/10.1046/j.1523-1755.2000.00216.x
Matsuzaki T, Yaguchi T, Shimizu K, Kita A, Ishibashi K, Takata K (2017) The distribution and function of aquaporins in the kidney: resolved and unresolved questions. Anat Sci Int 92(2):187–199. https://doi.org/10.1007/s12565-016-0325-2
Pallone TL, Kishore BK, Nielsen S, Agre P, Knepper MA (1997) Evidence that aquaporin-1 mediates NaCl-induced water flux across descending vasa recta. Am J Phys 272(5 Pt 2):F587–F596. https://doi.org/10.1152/ajprenal.1997.272.5.F587
Kandemir FM, Yildirim S, Caglayan C, Kucukler S, Eser G (2019) Protective effects of zingerone on cisplatin-induced nephrotoxicity in female rats. Environ Sci Pollut Res Int 26(22):22562–22574. https://doi.org/10.1007/s11356-019-05505-3
Zhang X, Williams MC, Rentsendorj O, D’Agnillo F (2018) Reversible renal glomerular dysfunction in guinea pigs exposed to glutaraldehyde-polymerized cell-free hemoglobin. Toxicology 402:37–49
Moraes A, Magalhães V (2018) Renal tubular damage caused by cylindrospermopsin (cyanotoxin) in mice. Toxicol Lett 286:89–95
Li X, Chuang PY, D’Agati VD, Dai Y, Yacoub R, Fu J, Xu J, Taku O, Premsrirut PK, Holzman LB (2015) Nephrin preserves podocyte viability and glomerular structure and function in adult kidneys. J Am Soc Nephrol 26(10):2361–2377
Xu J, Lian LJ, Wu C, Wang XF, Fu WY, Xu LH (2008) Lead induces oxidative stress, DNA damage and alteration of p53, Bax and Bcl-2 expressions in mice. Food Chem Toxicol 46(5):1488–1494. https://doi.org/10.1016/j.fct.2007.12.016
Caglayan C, Kandemir FM, Yıldırım S, Kucukler S, Kılınc MA, Saglam YS (2018) Zingerone ameliorates cisplatin-induced ovarian and uterine toxicity via suppression of sex hormone imbalances, oxidative stress, inflammation and apoptosis in female wistar rats. Biomed Pharmacother 102:517–530
Paulis MG, Hassan OA, Abbass MF, Mohammad MAH (2018) Structural and lipid peroxidation effects of lead on rat hippocampus and its attenuation by hydrogen rich water. J Chem Neuroanat 91:55–62. https://doi.org/10.1016/j.jchemneu.2018.04.004
Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L (2014) The role of oxidative stress during inflammatory processes. Biol Chem 395(2):203–230. https://doi.org/10.1515/hsz-2013-0241
Khalil SR, Mohammed WA, Zaglool AW, Elhady WM, Farag MR, El Sayed SAM (2019) Inflammatory and oxidative injury is induced in cardiac and pulmonary tissue following fipronil exposure in Japanese quail: mRNA expression of the genes encoding interleukin 6, nuclear factor kappa B, and tumor necrosis factor-alpha. Environ Pollut 251:564–572. https://doi.org/10.1016/j.envpol.2019.05.012
Turillazzi E, Neri M, Cerretani D, Cantatore S, Frati P, Moltoni L, Busardo FP, Pomara C, Riezzo I, Fineschi V (2016) Lipid peroxidation and apoptotic response in rat brain areas induced by long-term administration of nandrolone: the mutual crosstalk between ROS and NF-kB. J Cell Mol Med 20(4):601–612
Kaulmann A, Legay S, Schneider YJ, Hoffmann L, Bohn T (2016) Inflammation related responses of intestinal cells to plum and cabbage digesta with differential carotenoid and polyphenol profiles following simulated gastrointestinal digestion. Mol Nutr Food Res 60(5):992–1005
Liu B, Zhang H, Tan X, Yang D, Lv Z, Jiang H, Lu J, Baiyun R, Zhang Z (2017) GSPE reduces lead-induced oxidative stress by activating the Nrf2 pathway and suppressing miR153 and GSK-3β in rat kidney. Oncotarget 8(26):42226–42237
Gargouri M, Magné C, Dauvergne X, Ksouri R, El Feki A, Metges M-AG, Talarmin H (2013) Cytoprotective and antioxidant effects of the edible halophyte Sarcocornia perennis L.(swampfire) against lead-induced toxicity in renal cells. Ecotoxicol Environ Saf 95:44–51
Rehman MU, Tahir M, Khan AQ, Khan R, Lateef A, Qamar W, Ali F, Sultana S (2013) Chrysin suppresses renal carcinogenesis via amelioration of hyperproliferation, oxidative stress and inflammation: plausible role of NF-κB. Toxicol Lett 216(2-3):146–158
Kandemir F, Kucukler S, Eldutar E, Caglayan C, Gülçin I (2017) Chrysin protects rat kidney from paracetamol-induced oxidative stress, inflammation, apoptosis, and autophagy: a multi-biomarker approach. Sci Pharm 85(1):4
Esplugas R, LLovet MI, Bellés M, Serra N, Vallvé JC, Domingo JL, Linares V (2018) Renal and hepatic effects following neonatal exposure to low doses of Bisphenol-A and 137Cs. Food Chem Toxicol 114:270–277
Caglayan C, Temel Y, Kandemir FM, Yildirim S, Kucukler S (2018) Naringin protects against cyclophosphamide-induced hepatotoxicity and nephrotoxicity through modulation of oxidative stress, inflammation, apoptosis, autophagy, and DNA damage. Environ Sci Pollut Res 25(21):20968–20984
Rani N, Bharti S, Bhatia J, Tomar A, Nag T, Ray R, Arya DS (2015) Inhibition of TGF-β by a novel PPAR-γ agonist, chrysin, salvages β-receptor stimulated myocardial injury in rats through MAPKs-dependent mechanism. Nutr Metab 12(1):11
Gurer H, Ozgunes H, Neal R, Spitz DR, Ercal N (1998) Antioxidant effects of N-acetylcysteine and succimer in red blood cells from lead-exposed rats. Toxicology 128(3):181–189. https://doi.org/10.1016/s0300-483x(98)00074-2
Flora SJ, Pande M, Mehta A (2003) Beneficial effect of combined administration of some naturally occurring antioxidants (vitamins) and thiol chelators in the treatment of chronic lead intoxication. Chem Biol Interact 145(3):267–280. https://doi.org/10.1016/s0009-2797(03)00025-5
Gautam P, Flora SJ (2010) Oral supplementation of gossypin during lead exposure protects alteration in heme synthesis pathway and brain oxidative stress in rats. Nutrition 26(5):563–570. https://doi.org/10.1016/j.nut.2009.06.008
Flora SJ, Pachauri V (2010) Chelation in metal intoxication. Int J Environ Res Public Health 7(7):2745–2788. https://doi.org/10.3390/ijerph7072745
Fiati Kenston SS, Su H, Li Z, Kong L, Wang Y, Song X, Gu Y, Barber T, Aldinger J, Hua Q, Li Z, Ding M, Zhao J, Lin X (2018) The systemic toxicity of heavy metal mixtures in rats. Toxicol Res (Camb) 7(3):396–407. https://doi.org/10.1039/c7tx00260b
Xia D, Yu X, Liao S, Shao Q, Mou H, Ma W (2010) Protective effect of Smilax glabra extract against lead-induced oxidative stress in rats. J Ethnopharmacol 130(2):414–420
Aksu D, Sağlam Y, Yildirim S, Aksu T (2017) Effect of pomegranate (Punica granatum L.) juice on kidney, liver, heart and testis histopathological changes, and the tissues lipid peroxidation and antioxidant status in lead acetate-treated rats. Cell Mol Biol (Noisy le Grand) 63 (10)
Kwong WT, Friello P, Semba RD (2004) Interactions between iron deficiency and lead poisoning: epidemiology and pathogenesis. Sci Total Environ 330(1-3):21–37
Funding
This study was funded by the Unit of Scientific Research Projects in Munzur University. (Project No: YLMUB017-24)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kucukler, S., Benzer, F., Yildirim, S. et al. Protective Effects of Chrysin Against Oxidative Stress and Inflammation Induced by Lead Acetate in Rat Kidneys: a Biochemical and Histopathological Approach. Biol Trace Elem Res 199, 1501–1514 (2021). https://doi.org/10.1007/s12011-020-02268-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02268-8