Abstract
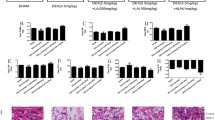
Aluminum (Al)-induced bone metabolism disorder is a primary cause of osteoporosis. Ginsenoside Rg3 (Rg3) has demonstrated therapeutic properties in the treatment of osteoporosis. The present study aimed to identify potential bone protection mechanisms of Rg3 against Al-induced osteoporosis in rats. In this study, forty healthy male Sprague-Dawley rats were randomly allocated into groups in which they were treated with AlCl3 (64 mg/kg/day) and/or Rg3 (20 mg/kg/day). AlCl3 was given orally to rats for 120 days, and from the 91st day, treated orally with Rg3 for 30 days. Rg3 attenuated AlCl3-induced accumulation of Al by decreasing the bone mineral density in the lumbar spines, femoral metaphysis, and tibia, and inhibited AlCl3-induced oxidative stress in rat bone by decreasing the levels of reactive oxygen species and malondialdehyde, while increasing glutathione peroxidase and superoxide dismutase activity. Rg3 facilitated bone formation by increasing the concentration of calcium, phosphorus, amino-terminal propeptide of type I procollagen, and carboxyl-terminal propeptide of type I procollagen, bone alkaline phosphatase activity in serum, and type I collagen, osteocalcin, and osteopontin protein expressions. Rg3 inhibited bone resorption by decreasing the content of N-terminal cross-linking telopeptide of type I collagen, C-terminal cross-linking telopeptide of type I collagen, and tartrate-resistant acid phosphatase 5b activity in serum. Rg3 promoted the mRNA expression of growth regulation factors by increasing transforming growth factor-β1, bone morphogenetic protein-2, insulin-like growth factor I, and core-binding factor α1. The results demonstrate that Rg3 can significantly attenuate Al accumulation, facilitate bone formation, inhibit bone resorption, resist oxidative stress, and promote the expression of factors that regulate growth. The results indicate that Rg3 is effective in alleviating AlCl3-induced osteoporosis.







Similar content being viewed by others
References
Oliveira V.M., Assis C.R.D., Costa H.M.S., Silva R.P.F., Santos J.F., Jr L.B.C., Bezerra R.S (2017) Aluminium sulfate exposure: a set of effects on hydrolases from brain, muscle and digestive tract of juvenile Nile tilapia ( Oreochromis niloticus ). Comp Biochem Physiol 191: 101–108
Wang F, Kang P, Li Z, Niu Q (2019) Role of MLL in the modification of H3K4me3 in aluminium-induced cognitive dysfunction. Chemosphere 232:121–129
Weidenhamer JD, Fitzpatrick MP, Biro AM, Kobunski PA, Hudson MR, Corbin RW, Gottesfeld P (2017) Metal exposures from aluminum cookware: an unrecognized public health risk in developing countries. Sci Total Environ 579:805–813
Nidheesh P.V., Khatri J., Anantha Singh T.S., Gandhimathi R., Ramesh S.T (2018) Review of zero-valent aluminium based water and wastewater treatment methods. Chemosphere 200: 621–631
Dam JWV, Trenfield MA, Harries SJ, Streten C, Harford AJ, Parry D, Dam RAV (2016) A novel bioassay using the barnacle Amphibalanus amphitrite to evaluate chronic effects of aluminium, gallium and molybdenum in tropical marine receiving environments. Mar Pollut Bull 112:427–435
Pagani S, Fini M, Giavaresi G, Salamanna F, Borsari V (2015) The active role of osteoporosis in the interaction between osteoblasts and bone metastases. Bone 79:176–182
Yuan FL, Xu RS, Jiang DL, He XL, Su Q, Jin C, Li X (2015) Leonurine hydrochloride inhibits osteoclastogenesis and prevents osteoporosis associated with estrogen deficiency by inhibiting the NF-κB and PI3K/Akt signaling pathways. Bone 75:128–137
Doğanlar ZB, Uzun M, Ovali MA, Dogan A, Ongoren G, Doğanlar O (2019) Melatonin attenuates caspase-dependent apoptosis in the thoracic aorta by regulating element balance and oxidative stress in pinealectomised rats. Appl Physiol Nutr Me 44:153–163
Yu Y, Zhou W, Zhou K, Liu W, Liang X, Chen Y, Sun D, Lin X (2018) Polyamines modulate aluminum-induced oxidative stress differently by inducing or reducing H2O2 production in wheat. Chemosphere 212:645–653
Li P, Luo W, Zhang H, Zheng X, Liu C, Ouyang H (2016) Effects of aluminum exposure on the bone stimulatory growth factors in rats. Bio Trace Elem Res 172:166–171
Kanazawa I, Canaff L, Abi RJ, Angrula A, Li J, Riddle RC, Boraschidiaz I, Komarova SV, Clemens TL, Murshed M (2015) Osteoblast menin regulates bone mass in vivo. J Biochem 290:3910–3924
Hu YC, Cheng HL, Hsieh BS, Huang LW, Huang TC, Chang KL (2012) Arsenic trioxide affects bone remodeling by effects on osteoblast differentiation and function. Bone 50:1406–1415
Song M, Huo H, Cao Z, Han Y, Gao L (2017) Aluminum trichloride inhibits the rat osteoblasts mineralization in vitro. Bio Trace Elem Res 175:186–193
Li X, Hu C, Zhu Y, Sun H, Li Y, Zhang Z (2011) Effects of aluminum exposure on bone mineral density, mineral, and trace elements in rats. Bio Trace Elem Res 143:378–385
Dénarié D, Constant E, Thomas T, Marotte H (2014) Could biomarkers of bone, cartilage or synovium turnover be used for relapse prediction in rheumatoid arthritis patients? Mediat Inflamm 2014:1–7
Duan X, Jin L, Zheng X, Zhe W, Zhang Y, Ying H, Yang T, Deng H (2016) Deficiency of ATP6V1H causes bone loss by inhibiting bone resorption and bone formation through the TGF-β1 pathway. Theranostics 6:2183–2195
Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, Aliprantis AO, Charles JF (2016) Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci 113:E7554–E7563
Lee J.H., Lee H.J., Yang M., Moon C., Kim J.C., Bae C.S., Jo S.K., Jang J.S., Kim S.H (2013) Effect of Korean red ginseng on radiation-induced bone loss in C3H/HeN mice. J Ginseng Res 37: 435–441
Kim J, Lee H, Kang KS, Chun KH, Hwang GS (2015) Protective effect of Korean red ginseng against glucocorticoid-induced osteoporosis in vitro and in vivo. J Ginseng Res 39:46–53
Yu-Mi S, Hyun-Joo J, Woo-Yong C, Chang-Jin L (2013) Antioxidative, anti-inflammatory, and matrix metalloproteinase inhibitory activities of 20(S)-ginsenoside Rg3 in cultured mammalian cell lines. Mol Biol Rep 40:269–279
Hanif S.M., Zubair S.M., Sungeun A., Kang S., Yeon-Ju K., Natarajan S., Yang D.U., Yang D.C (2013) Ginseng saponins and the treatment of osteoporosis: mini literature review. J Ginseng Res 37: 261–268
Siddiqi M.H., Siddiqi M.Z., Kang S., Noh H.Y., Ahn S., Simu S.Y., Aziz M.A., Sathishkumar N., Jiménez Pérez Z.E., Yang D.C (2015) Inhibition of osteoclast differentiation by ginsenoside Rg3 in RAW264.7 cells via RANKL, JNK and p38 MAPK pathways through a modulation of cathepsin K: an in Silico and in vitro study. Phytother Res 29: 1286–1294
Tian J, Fu F, Geng M, Jiang Y, Yang J, Jiang W, Wang C, Liu K (2005) Neuroprotective effect of 20( S )-ginsenoside Rg 3 on cerebral ischemia in rats. Neurosci Lett 374:92–97
Zhang X, Chen K, Wei B, Liu X, Lei Z, Bai X (2016) Ginsenosides Rg3 attenuates glucocorticoid-induced osteoporosis through regulating BMP-2/BMPR1A/Runx2 signaling pathway. Chem Biol Interact 256:188–197
Sun X, Liu J, Zhuang C, Yang X, Han Y, Shao B, Song M, Li Y, Zhu Y (2016) Aluminum trichloride induces bone impairment through TGF-β1/Smad signaling pathway. Toxicol 371:49–57
Yang X, Yu K, Wang H (2018) Bone impairment caused by AlCl3 is associated with activation of the JNK apoptotic pathway mediated by oxidative stress. Food ChemToxicol 116:307–314
Yang X, Zhang X, Zhang J, Ji Q, Huang W, Zhang X, Li Y (2019) Spermatogenesis disorder caused by T-2 toxin is associated with germ cell apoptosis mediated by oxidative stress. Environ Pollut 251:372–379
Fewtrell MS, Edmonds CJ, Isaacs E, Bishop NJ, Lucas A (2011) Aluminium exposure from parenteral nutrition in preterm infants and later health outcomes during childhood and adolescence. P Nutr Soc 70:299–304
EFSA Panel on Food additives f., processing aids, food m.i.c.w (2008) Safety of aluminium from dietary intake - scientific opinion of the panel on food additives, flavourings, processing aids and food contact materials (AFC). EFSA J 6: 1–34
Li P, Luo W, Zhang H, Zheng X, Liu C, Ouyang H (2015) Effects of aluminum exposure on the bone stimulatory growth factors in rats. Bio Trace Elem Res 172:166–171
Ammann P, Brennan TC, Mekraldi S, Aubert ML, Rizzoli R (2010) Administration of growth hormone in selectively protein-deprived rats decreases BMD and bone strength. Bone 46:1574–1581
Shibata Y, Ohsawa I, Watanabe T, Miura T, Sato Y (2003) Effects of physical training on bone mineral density and bone metabolism. J Physiol Anthropol Applied Hum Sci 22:203–208
Xu F, Wang P, Yao Q, Shao B, Yu H, Yu K, Li Y (2019) Lycopene alleviates AFB1-induced immunosuppression by inhibiting oxidative stress and apoptosis in the spleen of mice. Food Funct 10:3868–3879
Zhang C., Li X.-N., Xiang L.-R., Qin L., Lin J., Li J.L (2017) Atrazine triggers hepatic oxidative stress and apoptosis in quails (Coturnix C. coturnix) via blocking Nrf2-mediated defense response. Ecotox Environ Safe 137: 49–56
Zhang B, Guo H, Yang W, Li M, Zou Y, Loor JJ, Xia C, Xu C (2019) Effects of ORAI calcium release-activated calcium modulator 1 (ORAI1) on neutrophil activity in dairy cows with subclinical hypocalcemia1. J Anim Sci 97:3326–3336
Kwiecień S, Brzozowski T, Pch K, Konturek SJ (2020) The role of reactive oxygen species in action of nitric oxide-donors on stress-induced gastric mucosal lesions. J Physiol Pha 53:761–773
Roomruangwong C, Barbosa DS, de Farias CC, Matsumoto AK, Thl B, Morelli NR, Kanchanatawan B, Duleu S, Geffard M, Maes M (2018) Natural regulatory IgM-mediated autoimmune responses directed against malondialdehyde regulate oxidative and nitrosative pathways and coupled with IgM responses to nitroso-adducts attenuate depressive and physiosomatic symptoms at the end of term pregnancy. Psychiatry Clin Neurosci 72:116–130
Zhen T, Cheng J, Qian L, Lin Z, Kenny J, Tao W, Lin X, Yuan J, Quinn JMW, Tickner J (2017) Neohesperidin suppresses osteoclast differentiation, bone resorption and ovariectomised-induced osteoporosis in mice. Mol Cell Endocrinol 439:369–378
Cheng L, Yan S, Jiang Z, Cai P, Wang T, Zheng S (2017) Exercise enhance the ectopic bone formation of calcium phosphate biomaterials in muscles of mice. Mat Sci Eng C 77:136–141
Komaba H, Kaludjerovic J, Hu DZ, Nagano K, Amano K, Ide N, Sato T, Densmore MJ, Hanai JI, Olauson H (2017) Klotho expression in osteocytes regulates bone metabolism and controls bone formation. Kidney Int 92:599–611
Leeming DJ, Larsen DV, Zhang C, Hi Y, Veidal SS, Nielsen RH, Henriksen K, Zheng Q, Barkholt V, Riis BJ (2010) Enzyme-linked immunosorbent serum assays (ELISAs) for rat and human N-terminal pro-peptide of collagen type I (PINP)--assessment of corresponding epitopes. Clin Biochem 43:1249–1256
Linder CH, Ek-Rylander B, Krumpel M, Norgård M, Narisawa S, Millán JL, Andersson G, Magnusson P (2017) Bone alkaline phosphatase and tartrate-resistant acid phosphatase: potential co-regulators of bone mineralization. Calcified Tissue Int 101:92–101
Lian F, Zhao C, Qu J, Lian Y, Cui Y, Shan L, Yan J (2018) Icariin attenuates titanium particle-induced inhibition of osteogenic differentiation and matrix mineralization via miR-21-5p. Cell Bio Int 42:931–939
De FC, Messina A, Monda V, Viggiano E, Moscatelli F, Valenzano A, Esposito T, Sergio C, Cibelli G, Monda M (2017) Osteopontin: relation between adipose tissue and bone homeostasis. Stem Cells Int 2017:1–6
Moghaddam A, Müller U, Roth HJ, Wentzensen A, Grützner PA, Zimmermann G (2011) TRACP 5b and CTX as osteological markers of delayed fracture healing. Injury 42:758–764
Sun X, Jia H, Xu Q, Zhao C, Xu C (2019) Lycopene alleviates H2O2-induced oxidative stress, inflammation and apoptosis in bovine mammary epithelial cells via the NFE2L2 signaling pathway. Food Funct 10:6276–6285
Ripamonti U, Parak R, Klar RM, Dickens C, Dix-Peek T, Duarte R (2016) The synergistic induction of bone formation by the osteogenic proteins of the TGF-β supergene family. Biomaterials 104:279–296
Alvarez J, Horton J, Sohn P, Serra R (2001) The perichondrium plays an important role in mediating the effects of TGF-β1 on endochondral bone formation. Dev Dynam 221:311–321
Mbalaviele G, Sheikh S, Stains JP, Salazar VS, Cheng SL, Chen D, Civitelli R (2005) Beta-catenin and BMP-2 synergize to promote osteoblast differentiation and new bone formation. J Cell Biochem 94:403–418
Sakata T, Halloran BP, Elalieh HZ, Munson SJ, Rudner L, Venton L, Ginzinger D, Rosen CJ, Bikle DD (2003) Skeletal unloading induces resistance to insulin-like growth factor I on bone formation. Bone 32:669–680
Dai Z, Guo F, Wu F, Xu H, Yang C, Li J, Liang P, Zhang H, Qu L, Tan Y (2014) Integrin αvβ3 mediates the synergetic regulation of core-binding factor α1 transcriptional activity by gravity and insulin-like growth factor-1 through phosphoinositide 3-kinase signaling. Bone 69:126–132
Duan X, Xu H, Wang Y, Wang H, Li G, Jing L (2014) Expression of core-binding factor α1 and osteocalcin in fluoride-treated fibroblasts and osteoblasts. Theranostics 6:2183–2195
Funding
The study was supported by a grant from the National Science Foundation Project (31872530).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The design and experimental procedures of the animal studies were approved by the Animal Ethics Committee of the Northeast Agricultural University (Harbin, China).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Song, M., Jia, F., Cao, Z. et al. Ginsenoside Rg3 Attenuates Aluminum-Induced Osteoporosis Through Regulation of Oxidative Stress and Bone Metabolism in Rats. Biol Trace Elem Res 198, 557–566 (2020). https://doi.org/10.1007/s12011-020-02089-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02089-9